
Surface tension of a liquid is due to:
A. gravitational force between molecules
B. electrical force between molecules
C. adhesive force between molecules
D. cohesive force between the molecules
Answer
591.9k+ views
Hint: Surface tension is a property of the liquid surface acting as if it were a stretched elastic membrane. Surface tension in a liquid owes to the fact that the molecules attract one another, as each molecule forms a bond with the ones present in its vicinity. This inward net force causes the liquid molecules on the surface to contract and to resist being stretched or broken.
Complete step by step answer:
Surface tension is described as the tendency of liquid surfaces to shrink into the minimum possible surface area. At the liquid - air interfaces, surface tension is the result of the relatively greater attraction of liquid molecules to each other, due to cohesion, than to the molecules in the air, due to adhesion.
There are two primary mechanisms responsible for the existence of surface tension:
Inward force – It is present on the surface molecules of the liquid, causing it to contract.
Tangential force – It is present parallel to the surface of the liquid. The net effect of tangential force is that the liquid behaves as if its surface is covered with a stretched elastic membrane.
Surface tension has the dimension of force per unit length, or energy per unit area. The two dimensions are equivalent, but when we refer to energy per unit of area, it is obvious to use the term surface energy, which is a more general term in the sense that it applies to solids as well when we are defining surface tension.
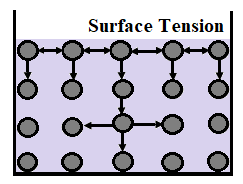
The presence of cohesive forces between the liquid molecules, are responsible for the phenomenon known as surface tension. The molecules located at the surface do not have other like molecules on all sides of them, and as a result, they cohere more strongly to those molecules which are directly associated with them on the surface.
Surface tension of a liquid is due to cohesive force between the molecules.
So, the correct answer is “Option D”.
Note:
Surface tension can be defined as the property of a liquid's surface, which allows it to resist an external force due to the cohesive nature of the liquid molecules. In materials science, surface tension is defined as either surface stress or surface energy. Surface tension allows insects, usually denser than water, to float and slide on the water surface.
Complete step by step answer:
Surface tension is described as the tendency of liquid surfaces to shrink into the minimum possible surface area. At the liquid - air interfaces, surface tension is the result of the relatively greater attraction of liquid molecules to each other, due to cohesion, than to the molecules in the air, due to adhesion.
There are two primary mechanisms responsible for the existence of surface tension:
Inward force – It is present on the surface molecules of the liquid, causing it to contract.
Tangential force – It is present parallel to the surface of the liquid. The net effect of tangential force is that the liquid behaves as if its surface is covered with a stretched elastic membrane.
Surface tension has the dimension of force per unit length, or energy per unit area. The two dimensions are equivalent, but when we refer to energy per unit of area, it is obvious to use the term surface energy, which is a more general term in the sense that it applies to solids as well when we are defining surface tension.

The presence of cohesive forces between the liquid molecules, are responsible for the phenomenon known as surface tension. The molecules located at the surface do not have other like molecules on all sides of them, and as a result, they cohere more strongly to those molecules which are directly associated with them on the surface.
Surface tension of a liquid is due to cohesive force between the molecules.
So, the correct answer is “Option D”.
Note:
Surface tension can be defined as the property of a liquid's surface, which allows it to resist an external force due to the cohesive nature of the liquid molecules. In materials science, surface tension is defined as either surface stress or surface energy. Surface tension allows insects, usually denser than water, to float and slide on the water surface.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




