
Sulphurous acid turns -------.
A.Blue litmus into red
B.Red litmus into blue
C.No change to any of the litmus paper
D.Can’t say
Answer
590.7k+ views
Hint: Litmus paper can be prepared from a filter paper. The filter paper has to be treated with a natural soluble dye called lichens. The piece of filter paper that is obtained is called litmus.
There are two types of litmus papers, blue litmus paper and red litmus paper.
Complete answer:
The given chemical in the question is sulphurous acid.
The molecular formula of sulphurous acid is\[{{H}_{2}}S{{O}_{3}}\].
The structure of the \[{{H}_{2}}S{{O}_{3}}\] is as follows
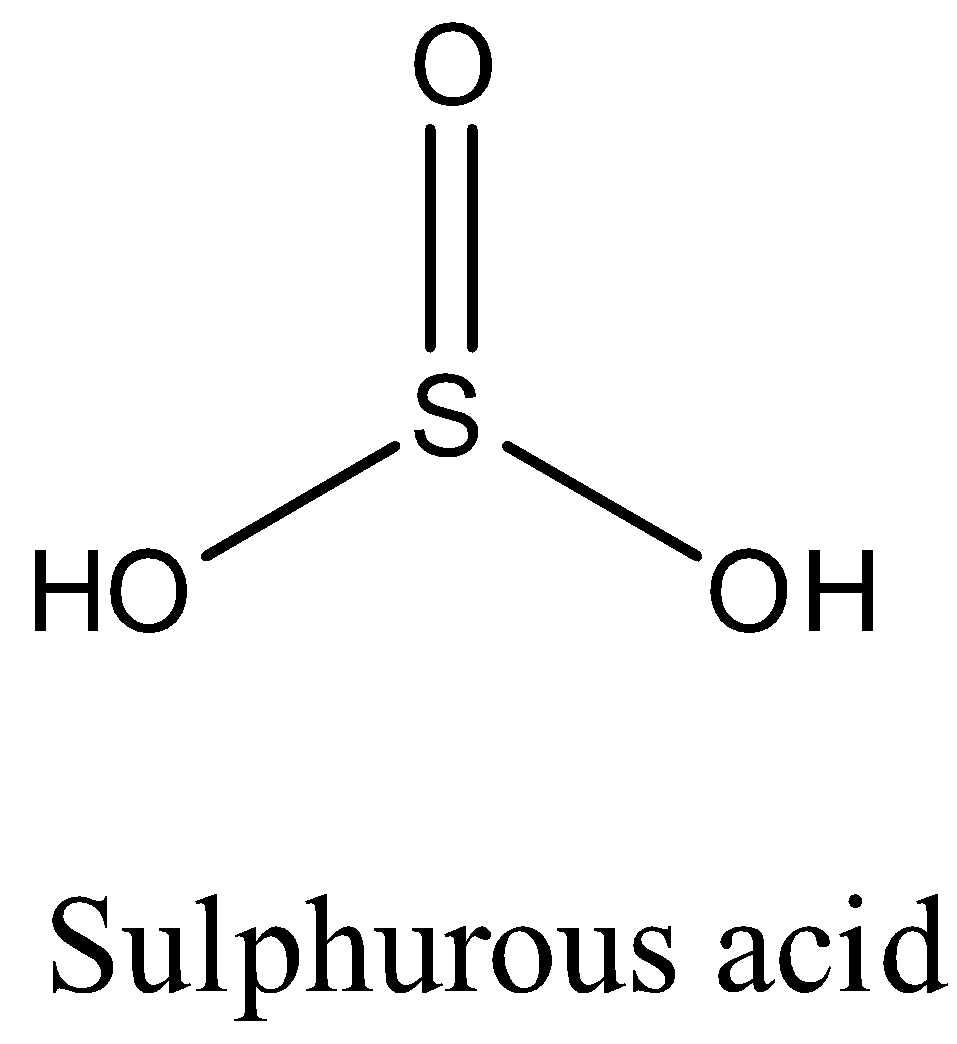
Sulphurous acid releases two hydrogens when dissolved in water then \[{{H}_{2}}S{{O}_{3}}\] is an acid.
Acid turns blue litmus to red litmus and base turns red litmus to blue litmus.
Therefore \[{{H}_{2}}S{{O}_{3}}\] turns blue litmus paper into red litmus paper.
So, the correct option is A.
Additional information:
Sulfurous acid is a weak acid and called dibasic acid also because it releases two hydrogens when dissolved in water.
Sulfurous acid and its respective salts are used as dominant reducing and disinfecting agents.
Sulfurous acid is used as a mild bleaching agent and can be used in place of chlorine to treat the materials that are sensitive towards chlorine.
Note:
Sulphurous acid can be prepared by treating sulphur dioxide with water.
\[S{{O}_{2}}+{{H}_{2}}O\to {{H}_{2}}S{{O}_{3}}\]
Sodium sulfite is the most important salt of Sulphurous acid. Sodium sulfite can be easily prepared by treating sulphurous acid with base.
\[{{H}_{2}}S{{O}_{3}}+2NaOH\to N{{a}_{2}}S{{O}_{3}}+2{{H}_{2}}O\]
Molecular formula of Sodium sulfite is \[N{{a}_{2}}S{{O}_{3}}\].
There are two types of litmus papers, blue litmus paper and red litmus paper.
Complete answer:
The given chemical in the question is sulphurous acid.
The molecular formula of sulphurous acid is\[{{H}_{2}}S{{O}_{3}}\].
The structure of the \[{{H}_{2}}S{{O}_{3}}\] is as follows

Sulphurous acid releases two hydrogens when dissolved in water then \[{{H}_{2}}S{{O}_{3}}\] is an acid.
Acid turns blue litmus to red litmus and base turns red litmus to blue litmus.
Therefore \[{{H}_{2}}S{{O}_{3}}\] turns blue litmus paper into red litmus paper.
So, the correct option is A.
Additional information:
Sulfurous acid is a weak acid and called dibasic acid also because it releases two hydrogens when dissolved in water.
Sulfurous acid and its respective salts are used as dominant reducing and disinfecting agents.
Sulfurous acid is used as a mild bleaching agent and can be used in place of chlorine to treat the materials that are sensitive towards chlorine.
Note:
Sulphurous acid can be prepared by treating sulphur dioxide with water.
\[S{{O}_{2}}+{{H}_{2}}O\to {{H}_{2}}S{{O}_{3}}\]
Sodium sulfite is the most important salt of Sulphurous acid. Sodium sulfite can be easily prepared by treating sulphurous acid with base.
\[{{H}_{2}}S{{O}_{3}}+2NaOH\to N{{a}_{2}}S{{O}_{3}}+2{{H}_{2}}O\]
Molecular formula of Sodium sulfite is \[N{{a}_{2}}S{{O}_{3}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




