
How many structures are possible for ${H_2}S{O_5}$?
Answer
507k+ views
Hint: ${H_2}S{O_5}$ is one of the strongest oxidants known $({E^0} = + 2.51V)$ and is highly explosive. Sulphur Oxoacid, another name for ${H_2}S{O_5}$ , is a special case where normal calculation gives you an oxidation state as $ + 8$ but Sulphur can only share a maximum $ + 6$ electrons. This is easily understood by drawing the compound structure.
So, the oxidation state of Sulphur is $ + 6$.
Complete answer:
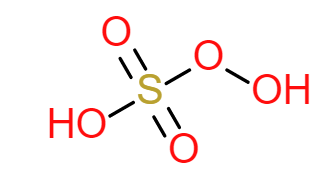
${H_2}S{O_5}$ being an inorganic acid only one structure is possible. It is a tetrahedral structure.
In this acid, the Sulphur center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula.
Like ${H_2}S{O_4}$ sulfur in a molecule is in a hybridized state. So it will adopt tetrahedral structure. Both ${H_2}S{O_4}$ and ${H_2}S{O_5}$ are in $ + 6$ oxidation state.
Compared with methane. Put sulfur as the central atom. Two apexes of the tetrahedron will be occupied by two-oxygen atoms (double bonded oxygen). One apex will be occupied by $ - OH$. Remaining will be occupied by peroxy bonds $( - O - OH)$.
It contains two double bonded oxygen atoms, which are attached to the Sulphur atom and two $ - OH$ groups.
Structure of ${H_2}S{O_5}$ is:
Note:
Peroxymonosulfuric acid, $({H_2}S{O_5})$, also known as persulfuric acid, peroxydisulfuric acid. Or Caro’s acid.
Disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure
It is used in laboratories as a last resort in removing organic material since ${H_2}S{O_5}$ can fully oxidize any organic materials
Sometimes, ${H_2}S{O_5}$ is confused with ${H_2}{S_2}{O_8}$, known as peroxydisulfuric acid.
So, the oxidation state of Sulphur is $ + 6$.
Complete answer:
${H_2}S{O_5}$ being an inorganic acid only one structure is possible. It is a tetrahedral structure.
In this acid, the Sulphur center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula.
Like ${H_2}S{O_4}$ sulfur in a molecule is in a hybridized state. So it will adopt tetrahedral structure. Both ${H_2}S{O_4}$ and ${H_2}S{O_5}$ are in $ + 6$ oxidation state.
Compared with methane. Put sulfur as the central atom. Two apexes of the tetrahedron will be occupied by two-oxygen atoms (double bonded oxygen). One apex will be occupied by $ - OH$. Remaining will be occupied by peroxy bonds $( - O - OH)$.
It contains two double bonded oxygen atoms, which are attached to the Sulphur atom and two $ - OH$ groups.
Structure of ${H_2}S{O_5}$ is:

Note:
Peroxymonosulfuric acid, $({H_2}S{O_5})$, also known as persulfuric acid, peroxydisulfuric acid. Or Caro’s acid.
Disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure
It is used in laboratories as a last resort in removing organic material since ${H_2}S{O_5}$ can fully oxidize any organic materials
Sometimes, ${H_2}S{O_5}$ is confused with ${H_2}{S_2}{O_8}$, known as peroxydisulfuric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




