
What is the structure of the molecule named $ m - $ dichlorobenzene?
Answer
495k+ views
Hint: The structural formula of a chemical compound is a graphic representation of the molecular structure determined by structural chemistry methods, showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bonding within the molecule is also shown, either explicitly or implicitly.
Complete answer:
We have to follow the following steps to find the shape of the molecule.
-Draw the Lewis Structure.
-Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
-Name the electron-group geometry.
-Looking at the positions of other atomic nuclei around the central part determines the molecular geometry.
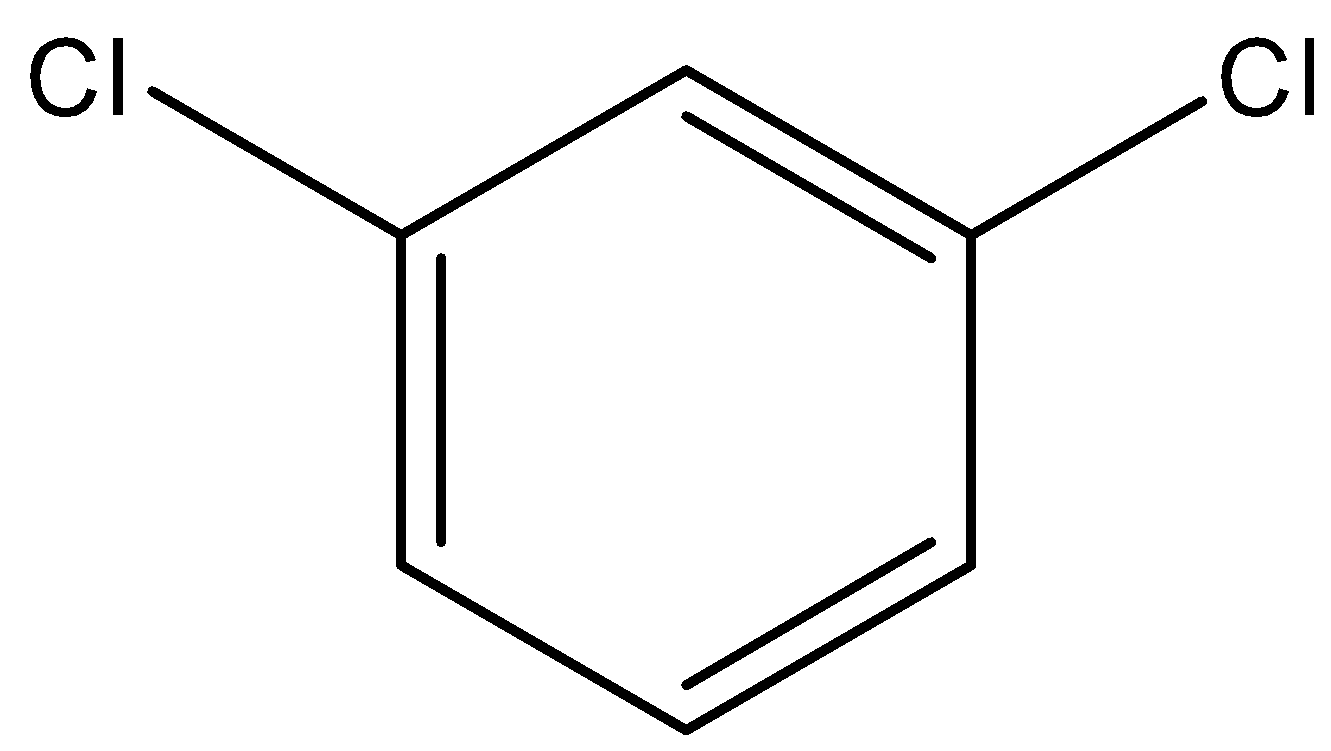
The prefix $ m - $ tells us that the two $ Cl $ atoms are on $ C - 1 $ and $ C - 3 $ of the benzene ring.
Now we put a $ Cl $ atom on any $ C $ atom of the benzene ring. Then, this becomes $ C - 1 $ . Then we count in any direction around the ring and put a $ Cl $ atom on $ C - 3 $ .
Additional Information:
Molecules are held together by shared electron pairs, or covalent bonds. Such bonds are directional, meaning that the atoms adopt specific positions relative to one another so as to maximize the bond strengths. As a result, each molecule has a definite, fairly rigid structure, or spatial distribution of its atoms.
Note:
$ 1,3 - $ Dichlorobenzene is the least common of the three isomers of dichlorobenzene, it is a colorless liquid that is insoluble in water. It is produced as a minor byproduct of the chlorination of benzene, but can also be prepared in a directed manner by the Sandmeyer reaction of $ 3 - $ chloroaniline. It also arises from the isomerization of the other dichlorobenzene at high temperature.
Complete answer:
We have to follow the following steps to find the shape of the molecule.
-Draw the Lewis Structure.
-Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
-Name the electron-group geometry.
-Looking at the positions of other atomic nuclei around the central part determines the molecular geometry.
The prefix $ m - $ tells us that the two $ Cl $ atoms are on $ C - 1 $ and $ C - 3 $ of the benzene ring.
Now we put a $ Cl $ atom on any $ C $ atom of the benzene ring. Then, this becomes $ C - 1 $ . Then we count in any direction around the ring and put a $ Cl $ atom on $ C - 3 $ .

Additional Information:
Molecules are held together by shared electron pairs, or covalent bonds. Such bonds are directional, meaning that the atoms adopt specific positions relative to one another so as to maximize the bond strengths. As a result, each molecule has a definite, fairly rigid structure, or spatial distribution of its atoms.
Note:
$ 1,3 - $ Dichlorobenzene is the least common of the three isomers of dichlorobenzene, it is a colorless liquid that is insoluble in water. It is produced as a minor byproduct of the chlorination of benzene, but can also be prepared in a directed manner by the Sandmeyer reaction of $ 3 - $ chloroaniline. It also arises from the isomerization of the other dichlorobenzene at high temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




