
What is the structure of $\text{P}{{\text{F}} _ {5}} $molecule?
Answer
538.2k+ views
Hint:We can calculate the structure or the geometry of the$\text{P}{{\text{F}} _ {5}} $molecule by knowing its hybridization (it is process of inter-mixing of the orbitals to form new orbitals of equivalent energy). The hybridization can be calculated by the formula:
H= $\dfrac {1}{2} $ (V+M-C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Identify the structure.
Complete step-by-step answer:To know the structure of any molecule, we should know its hybridization. By the term hybridization we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to fi8nd the hybridization of $\text{P}{{\text{F}} _ {5}} $molecule by the formula as:
H= $\dfrac {1}{2} $ (V+M-C+A) ---------(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
So, in case of $\text{P}{{\text{F}} _ {5}} $molecule; V in P=5
M in F=5
Since there is no charge on the $\text{P}{{\text{F}} _ {5}} $molecule, so both C and A=0
Put all these values in equation (1), we get
$H$=$\dfrac {1}{2} $(5+5)
$\Rightarrow H$=$\dfrac {1}{2} $(10)
$\therefore H=5$
So, the $\text{P}{{\text{F}} _ {5}} $involves the $\text{s}{{\text{p}} ^ {3}} \text{d}$ hybridization i.e. it has trigonal bipyramidal geometry or the structure and it involves the mixing of the one s, three p and one d-orbitals.
The geometry or structure of $\text{P}{{\text{F}}_{5}}$is as;
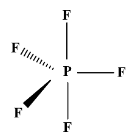
In this, there are five electrons in the valence shell of the excited phosphorus atom i.e. one electron in 3s orbital , three electrons in three p-orbitals and one electron in the d-orbital and can form five bonds with fluorine atom which requires only one electron to complete its valence shell. So, each of five fluorine atoms forms a bond with the phosphorus atoms thus resulting in the formation of five P-F bonds.
Note: For the orbitals which undergo hybridization, they should have only small differences in their energies and only those orbitals are hybridized which are present in the valence shell of the atom.
H= $\dfrac {1}{2} $ (V+M-C+A)
Here, V represents the number of electrons in the valence shell of the atom, M represents the monovalent atoms attached to that atom and C and A represents the charges of the cations and anions. Identify the structure.
Complete step-by-step answer:To know the structure of any molecule, we should know its hybridization. By the term hybridization we mean the phenomenon of inter-mixing of the orbitals of slightly different energies so as to redistribute their energies and to give a new set of orbitals of equivalent energies and shape.
First, we have to fi8nd the hybridization of $\text{P}{{\text{F}} _ {5}} $molecule by the formula as:
H= $\dfrac {1}{2} $ (V+M-C+A) ---------(1)
Here, V= number of the valence electrons in the atom, M= number of the monovalent atoms bonded to the central atom, C=the charge on the cation and A= the charge on the anion.
So, in case of $\text{P}{{\text{F}} _ {5}} $molecule; V in P=5
M in F=5
Since there is no charge on the $\text{P}{{\text{F}} _ {5}} $molecule, so both C and A=0
Put all these values in equation (1), we get
$H$=$\dfrac {1}{2} $(5+5)
$\Rightarrow H$=$\dfrac {1}{2} $(10)
$\therefore H=5$
So, the $\text{P}{{\text{F}} _ {5}} $involves the $\text{s}{{\text{p}} ^ {3}} \text{d}$ hybridization i.e. it has trigonal bipyramidal geometry or the structure and it involves the mixing of the one s, three p and one d-orbitals.
The geometry or structure of $\text{P}{{\text{F}}_{5}}$is as;

In this, there are five electrons in the valence shell of the excited phosphorus atom i.e. one electron in 3s orbital , three electrons in three p-orbitals and one electron in the d-orbital and can form five bonds with fluorine atom which requires only one electron to complete its valence shell. So, each of five fluorine atoms forms a bond with the phosphorus atoms thus resulting in the formation of five P-F bonds.
Note: For the orbitals which undergo hybridization, they should have only small differences in their energies and only those orbitals are hybridized which are present in the valence shell of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




