
Structure of ${N_2}O$ is similar to that of $C{O_2}$ .
A. True
B. False
Answer
578.7k+ views
Hint: Structures of simple molecules can be deduced by using some simple rules given by Lewis.
Complete step by step answer:
We know that the electronic theory of chemical bonding was given by Kössel and Lewis and it is also known as octet rule for according to this theory atoms combine to complete their octet in valence shell. Furthermore, covalent bonding was explained on the similar lines as formation of bond by sharing of valence electrons. To explain this Lewis dot structures were used in which valence electrons are represented by dots.
We can write the Lewis structure for a given molecule by following some simple steps as follows:
Let’s take a simple example of an oxygen molecule which has the formula ${O_2}$ .
Firstly, we would calculate the total number of valence electrons contributed by all the constituting atoms. So, in molecule ${O_2}$, each oxygen atom would contribute its six valence electrons giving a total of twelve electrons for the Lewis structure.
If there is a charge as well, we would incorporate that as well. In molecules, there is no charge.
Now we would write the skeletal structure of the molecule by using the symbols of the atoms and single bonds.
Lastly, remaining electrons would be used for multiple bonding or taken as lone pairs.
Let’s write the Lewis structure for ${N_2}O$ by using the above steps. In ${N_2}O$, we can calculate the total valence electrons as follows:
$\begin{array}{c}
{N_2}O = \left\{ {2 \times \left( 5 \right)} \right\} + 6\\
= 10 + 6\\
= 16
\end{array}$
Now, we can write the skeletal structure of ${N_2}O$ as follows:
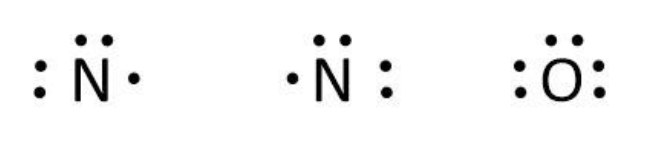
We can use the valence electrons for bonding and remaining as lone pairs as follows:
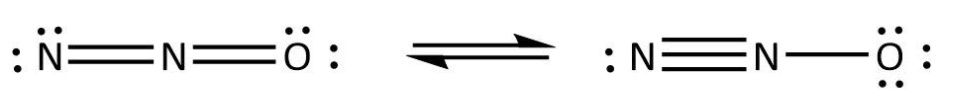
Similarly, we write the Lewis structure for $C{O_2}$ by using the same steps. In $C{O_2}$, we can calculate the total valence electrons as follows:
$\begin{array}{c}
C{O_2} = 4 + \left\{ {2 \times \left( 6 \right)} \right\}\\
= 4 + 12\\
= 16
\end{array}$
Now, we can write the skeletal structure of $C{O_2}$ as follows:
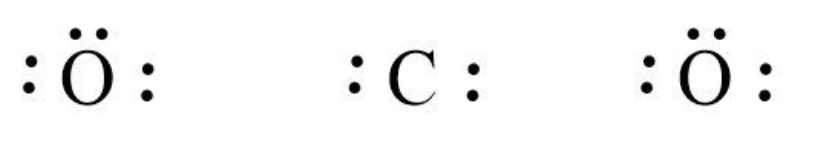
We can use the valence electrons for bonding and remaining as lone pairs as follows:
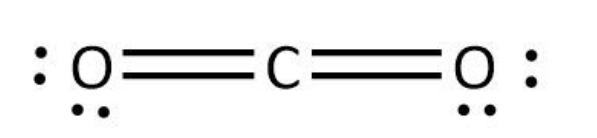
So, we can see that both the structures are linear. Hence, the given statement is true.
Hence, the correct option is (A).
Note:
We need to calculate the valence electrons carefully and distribute them as per the valency of the atom.
Complete step by step answer:
We know that the electronic theory of chemical bonding was given by Kössel and Lewis and it is also known as octet rule for according to this theory atoms combine to complete their octet in valence shell. Furthermore, covalent bonding was explained on the similar lines as formation of bond by sharing of valence electrons. To explain this Lewis dot structures were used in which valence electrons are represented by dots.
We can write the Lewis structure for a given molecule by following some simple steps as follows:
Let’s take a simple example of an oxygen molecule which has the formula ${O_2}$ .
Firstly, we would calculate the total number of valence electrons contributed by all the constituting atoms. So, in molecule ${O_2}$, each oxygen atom would contribute its six valence electrons giving a total of twelve electrons for the Lewis structure.
If there is a charge as well, we would incorporate that as well. In molecules, there is no charge.
Now we would write the skeletal structure of the molecule by using the symbols of the atoms and single bonds.
Lastly, remaining electrons would be used for multiple bonding or taken as lone pairs.
Let’s write the Lewis structure for ${N_2}O$ by using the above steps. In ${N_2}O$, we can calculate the total valence electrons as follows:
$\begin{array}{c}
{N_2}O = \left\{ {2 \times \left( 5 \right)} \right\} + 6\\
= 10 + 6\\
= 16
\end{array}$
Now, we can write the skeletal structure of ${N_2}O$ as follows:
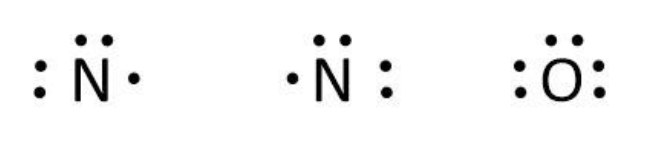
We can use the valence electrons for bonding and remaining as lone pairs as follows:

Similarly, we write the Lewis structure for $C{O_2}$ by using the same steps. In $C{O_2}$, we can calculate the total valence electrons as follows:
$\begin{array}{c}
C{O_2} = 4 + \left\{ {2 \times \left( 6 \right)} \right\}\\
= 4 + 12\\
= 16
\end{array}$
Now, we can write the skeletal structure of $C{O_2}$ as follows:
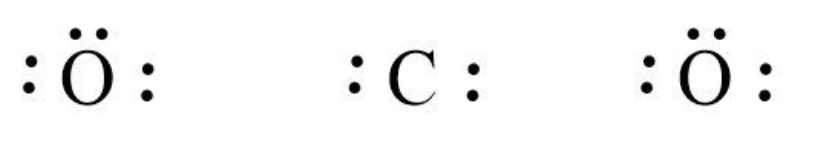
We can use the valence electrons for bonding and remaining as lone pairs as follows:
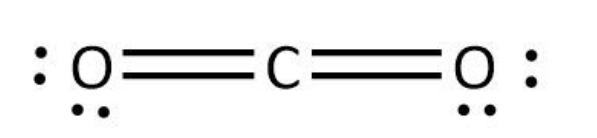
So, we can see that both the structures are linear. Hence, the given statement is true.
Hence, the correct option is (A).
Note:
We need to calculate the valence electrons carefully and distribute them as per the valency of the atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




