
What is the structure of diborane:
A. four $2c - 2e$ bonds four $3c - 2e$bonds
B. two $2c - 2e$ bonds and two $3c - 2e$ bonds
C. two $2c - 2e$ bonds and four $3c - 2e$bonds
D. four $2c - 2e$ bonds and two $3c - 2e$bonds
Answer
585.9k+ views
Hint: Diborane consists of boron and hydrogen elements with the molecular formula ${B_2}{H_6}$ . It is a colourless gas with a sweet odour.
Complete step by step answer: In Diborane, four terminal hydrogens and bridging hydrogens between the borons are present.
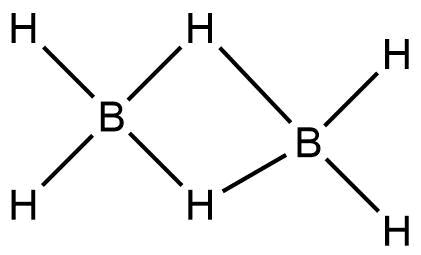
The bonds between the terminal hydrogens and boron is $2 - Center,2 - electron$ covalent bond.
Whereas, the bonds between boron and the bridging hydrogens are different. Each Boron atom uses its two electrons in bonding with the terminal hydrogen. The remaining one valence electron participates in bridging. The Boron and bridging hydrogen provide one electron each and form a$B{}_2{H_2}$ ring, held by four electrons forming two $3-$Centre\[ - 2\] electron
So, the correct answer is “Option D”.
Additional Information: The length of the B (terminal) bond is \[1.19{\text{ }}A\], whereas the length of the $B - H$(Bridges) Bond is \[1.33A\]. Due to difference in bond length, bond strength is also different. The $B - H$(Bridge) bond is relatively weaker than the $B - H$(terminal) bond.
Note: This type of bond is known as "Banana bond". Gallium also forms a similar compound $G{a_2}{H_6}$ , known as "digauane".
Complete step by step answer: In Diborane, four terminal hydrogens and bridging hydrogens between the borons are present.

The bonds between the terminal hydrogens and boron is $2 - Center,2 - electron$ covalent bond.
Whereas, the bonds between boron and the bridging hydrogens are different. Each Boron atom uses its two electrons in bonding with the terminal hydrogen. The remaining one valence electron participates in bridging. The Boron and bridging hydrogen provide one electron each and form a$B{}_2{H_2}$ ring, held by four electrons forming two $3-$Centre\[ - 2\] electron
So, the correct answer is “Option D”.
Additional Information: The length of the B (terminal) bond is \[1.19{\text{ }}A\], whereas the length of the $B - H$(Bridges) Bond is \[1.33A\]. Due to difference in bond length, bond strength is also different. The $B - H$(Bridge) bond is relatively weaker than the $B - H$(terminal) bond.
Note: This type of bond is known as "Banana bond". Gallium also forms a similar compound $G{a_2}{H_6}$ , known as "digauane".
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




