
Structure formula of ethyne is –
(A) \[H-C\equiv C-C{{H}_{3}}\]
(B) \[H-C\equiv C-H\]
(C))
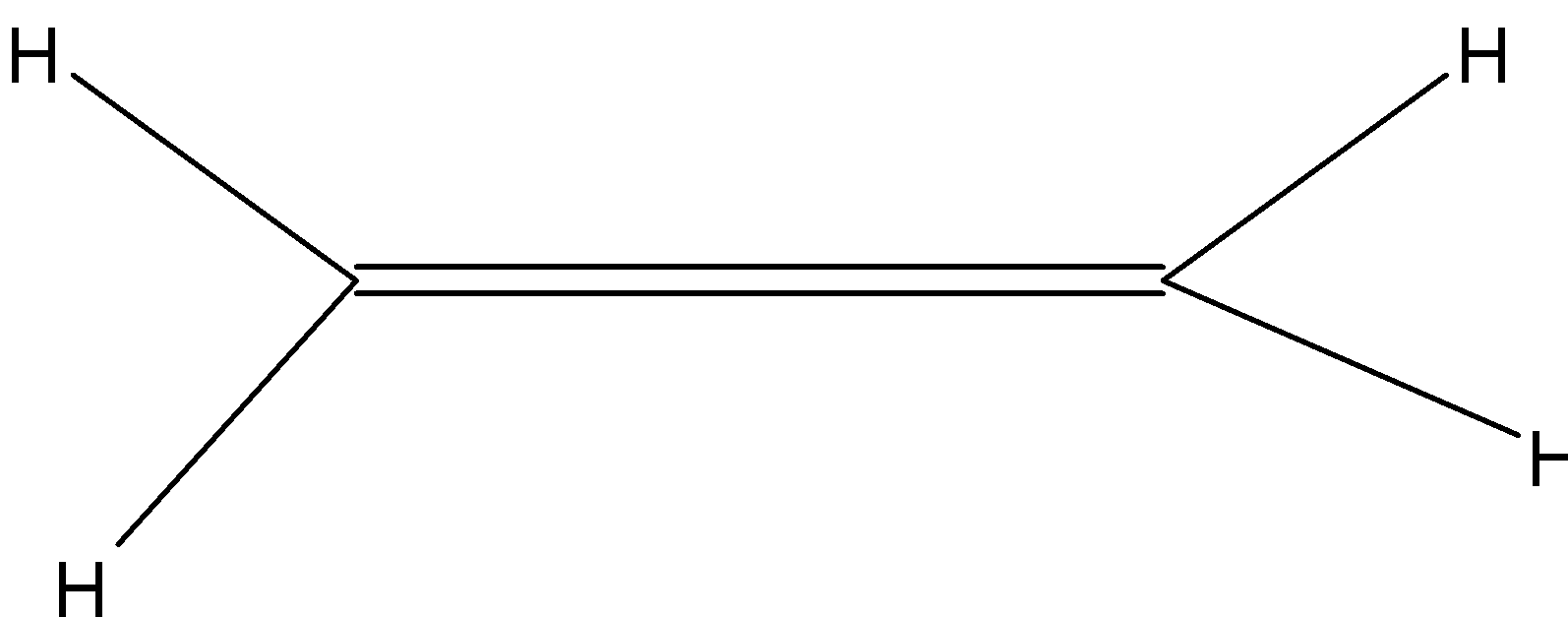
(D)
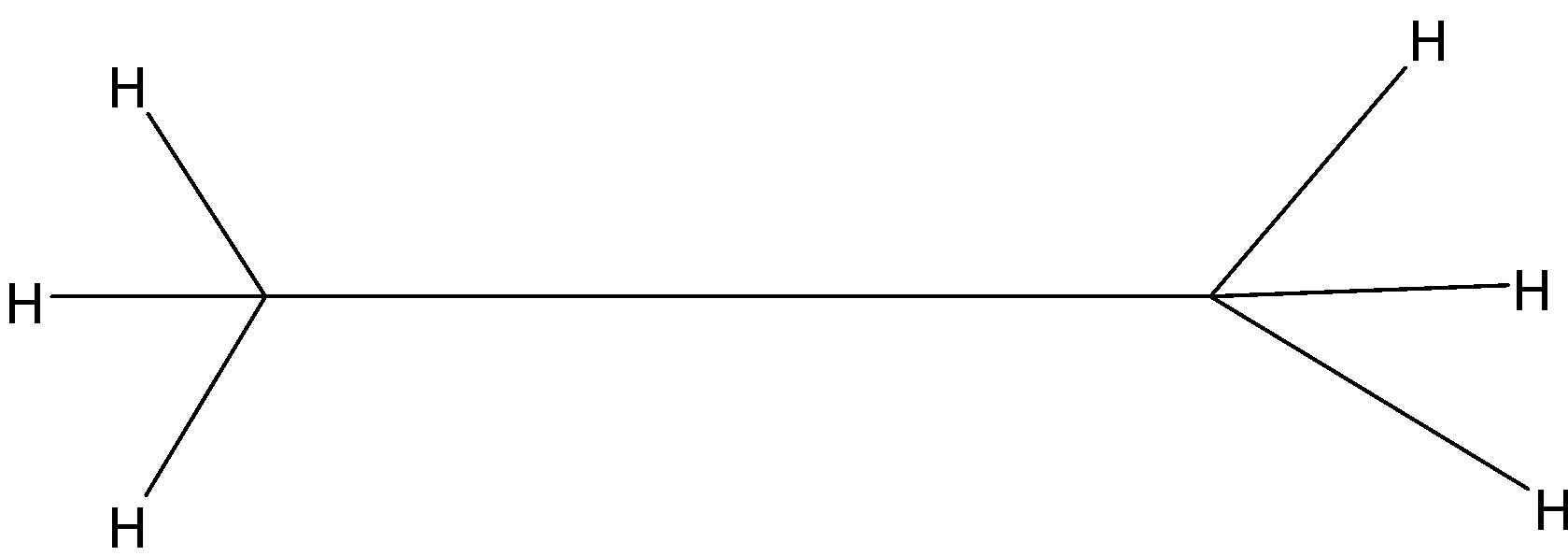


Answer
598.2k+ views
Hint: In this question IUPAC name of an organic compound is given and we have to find the chemical formula and structure of the given compound by using rules of IUPAC.
Complete step by step solution:
> We know that The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space and chemical formula that shows how the atoms making up a compound are arranged within the molecule. By applying the rules of IUPAC naming or structure from IUPAC naming we can find the structure of ethyne.
Here, ethyne = eth + yne
> So, here we know that in ethyne ‘eth’ is a prefix and ‘yne’ is a suffix. So, from the suffix ‘yne’ we can say that this organic compound has a triple bond in its structure. And from the ‘eth’ prefix we can say that it has two carbon atoms in its parent chain. In this compound there is not any type of naming of substituents, so we can say there will be no substituents in the structural formula of ethyne.
So, from the above explanation we can make the below given structure:
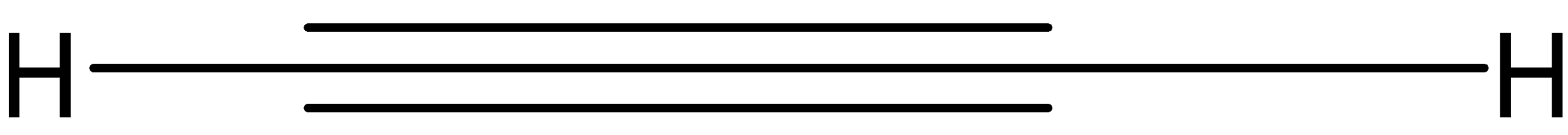
And the chemical formula is:
\[HC\equiv CH\]
So, the correct answer is option “B”.
Note: In this compound degree of unsaturation every carbon is one. And you should remember that suffix ‘ane’ is used for all single bonds, ‘ene’ is used for double bond and ‘yne’ is used for triple bond.
Complete step by step solution:
> We know that The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space and chemical formula that shows how the atoms making up a compound are arranged within the molecule. By applying the rules of IUPAC naming or structure from IUPAC naming we can find the structure of ethyne.
Here, ethyne = eth + yne
> So, here we know that in ethyne ‘eth’ is a prefix and ‘yne’ is a suffix. So, from the suffix ‘yne’ we can say that this organic compound has a triple bond in its structure. And from the ‘eth’ prefix we can say that it has two carbon atoms in its parent chain. In this compound there is not any type of naming of substituents, so we can say there will be no substituents in the structural formula of ethyne.
So, from the above explanation we can make the below given structure:
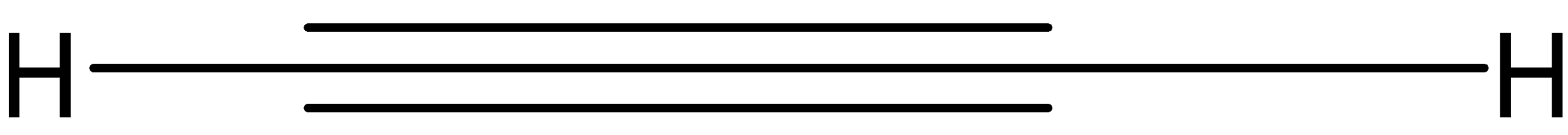
And the chemical formula is:
\[HC\equiv CH\]
So, the correct answer is option “B”.
Note: In this compound degree of unsaturation every carbon is one. And you should remember that suffix ‘ane’ is used for all single bonds, ‘ene’ is used for double bond and ‘yne’ is used for triple bond.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




