
What is the structure for \[3,4 - \dim ethylpent - 4 - en - 1 - yne\] and \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] ?
Answer
487.5k+ views
Hint: The IUPAC name can be written by considering the root word from the longest carbon chain. The substituents must be written with positions also. If there were more than one functional group, then the alphabetical order should be followed to write the IUPAC nomenclature.
Complete answer:
Alkanes are the saturated hydrocarbons consisting of carbon-carbon single \[\left( {C - C} \right)\] bonds. Alkenes are the unsaturated hydrocarbons consisting of one or more carbon-carbon double \[\left( {C = C} \right)\] bonds. Alkenes are the unsaturated hydrocarbons consisting of one or more carbon-carbon triple \[\left( {C \equiv C} \right)\] bonds.
Given IUPAC name is \[3,4 - \dim ethylpent - 4 - en - 1 - yne\] which means there are two methyl groups at \[3,4\] positions, double bond at \[{4^{th}}\] position and triple bond at \[{1^{st}}\] position. The base chain which is the longest carbon chain has five carbon atoms. Thus, the root word is pent.
The structure for the IUPAC name of \[3,4 - \dim ethylpent - 4 - en - 1 - yne\] is
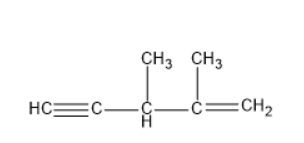
Given IUPAC name is \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] which means there are two methyl groups at \[2,3,\] positions, double bond at \[{1^{st}}\] position and triple bond at \[{4^{th}}\] position. The base chain which is the longest carbon chain has five carbon atoms. Thus, the root word is pent.
The structure for the IUPAC name of \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] is
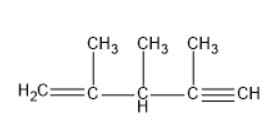
Both the above two structures were the same, but the numbering was done from alkene side in one structure and from alkyne side in another structure.
Note:
When any compound consists of two functional groups, the functional group that comes first in alphabetical order should be written first. Thus, in both the structures, the IUPAC name \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] is the correct one, as alkene comes first and has a lower numbering than alkynes.
Complete answer:
Alkanes are the saturated hydrocarbons consisting of carbon-carbon single \[\left( {C - C} \right)\] bonds. Alkenes are the unsaturated hydrocarbons consisting of one or more carbon-carbon double \[\left( {C = C} \right)\] bonds. Alkenes are the unsaturated hydrocarbons consisting of one or more carbon-carbon triple \[\left( {C \equiv C} \right)\] bonds.
Given IUPAC name is \[3,4 - \dim ethylpent - 4 - en - 1 - yne\] which means there are two methyl groups at \[3,4\] positions, double bond at \[{4^{th}}\] position and triple bond at \[{1^{st}}\] position. The base chain which is the longest carbon chain has five carbon atoms. Thus, the root word is pent.
The structure for the IUPAC name of \[3,4 - \dim ethylpent - 4 - en - 1 - yne\] is

Given IUPAC name is \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] which means there are two methyl groups at \[2,3,\] positions, double bond at \[{1^{st}}\] position and triple bond at \[{4^{th}}\] position. The base chain which is the longest carbon chain has five carbon atoms. Thus, the root word is pent.
The structure for the IUPAC name of \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] is

Both the above two structures were the same, but the numbering was done from alkene side in one structure and from alkyne side in another structure.
Note:
When any compound consists of two functional groups, the functional group that comes first in alphabetical order should be written first. Thus, in both the structures, the IUPAC name \[2,3 - \dim ethylpent - 1 - en - 4 - yne\] is the correct one, as alkene comes first and has a lower numbering than alkynes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




