
Structural formula of ethyne is:
1.

2.

3.
4.
A. 2
B. 1
C. 4
D. 3




Answer
545.7k+ views
Hint: To find the structural formula of ethyne, you must understand the prefix and suffix of ethyne. We can conclude that ethyne is an alkyne, that is, an unsaturated hydrocarbon.
Complete step-by-step answer:We know that the word root eth- in a hydrocarbon represents the presence of 2 carbon atoms and the suffix –yne represents the presence of a triple bond between the carbon atoms.
Considering structure 1,
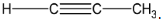
The given structural formula represents that of an alkyne. However, this alkyne contains three carbon atoms, whereas the number of carbon atoms in ethyne is 2. The given structure is that of propyne. Thus, it is not correct.
Considering structure 2,

The given structural formula represents that of an alkyne with two carbon atoms. Thus, it best represents the ethyne molecule.
Considering structure 3,
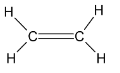
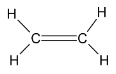
The number of carbon atoms in the molecule is 2. The given structural formula represents that of an alkene. The given structure molecule is Ethene. Thus it is not correct.
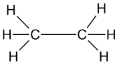
Considering structure 4,
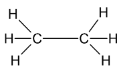
The number of carbon atoms in the molecule is 2. The given structural formula represents that of an alkane. The given structure molecule is Ethane. Thus it is not correct.
Thus, the correct option is A.
Note:Hydrocarbons can be classified widely into three categories which are alkanes, alkenes and alkynes. Alkanes are saturated organic compounds that contain only single bonds between all the carbon atoms. However, alkenes and alkynes are unsaturated compounds and there are double and triple bonds present between the carbon atoms, respectively, in addition to single bonds. This difference in the bonding in the carbon chain gives them different physical as well as chemical properties.
Complete step-by-step answer:We know that the word root eth- in a hydrocarbon represents the presence of 2 carbon atoms and the suffix –yne represents the presence of a triple bond between the carbon atoms.
Considering structure 1,
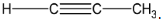
The given structural formula represents that of an alkyne. However, this alkyne contains three carbon atoms, whereas the number of carbon atoms in ethyne is 2. The given structure is that of propyne. Thus, it is not correct.
Considering structure 2,

The given structural formula represents that of an alkyne with two carbon atoms. Thus, it best represents the ethyne molecule.
Considering structure 3,

The number of carbon atoms in the molecule is 2. The given structural formula represents that of an alkene. The given structure molecule is Ethene. Thus it is not correct.
Considering structure 4,

The number of carbon atoms in the molecule is 2. The given structural formula represents that of an alkane. The given structure molecule is Ethane. Thus it is not correct.
Thus, the correct option is A.
Note:Hydrocarbons can be classified widely into three categories which are alkanes, alkenes and alkynes. Alkanes are saturated organic compounds that contain only single bonds between all the carbon atoms. However, alkenes and alkynes are unsaturated compounds and there are double and triple bonds present between the carbon atoms, respectively, in addition to single bonds. This difference in the bonding in the carbon chain gives them different physical as well as chemical properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




