
How many structural and geometrical isomers are possible for dimethyl cyclohexane?
A.3,6
B.4,6
C.6,4
D.3, 3
Answer
558.6k+ views
Hint: We know that isomerism is the phenomenon in which the compounds have the same chemical formula but their structure is different. There are two types of isomerism that is structural isomerism and stereoisomerism.
Complete step by step answer:
Let’s first discuss structural isomerism in detail. Structural isomerism is shown by the compounds having the same molecular formulae differing in the arrangement of the atoms. There are four possible geometrical isomers for dimethyl cyclohexane.
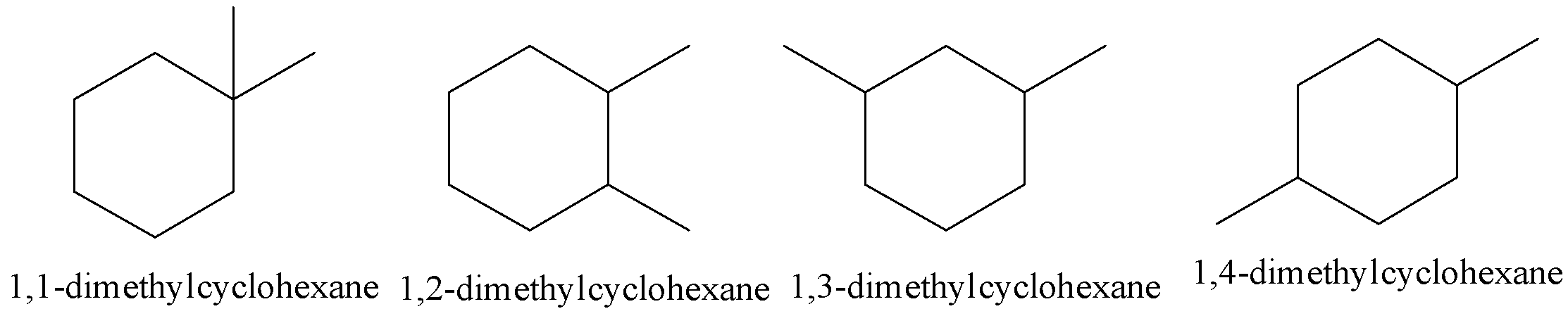
All the above structural isomers possess the same chemical formula but their structures are different.
Let’s discuss geometrical isomerism in detail. In geometrical isomerism, two types of isomers namely cis and trans present. Geometrical isomerism is a type of stereoisomerism.
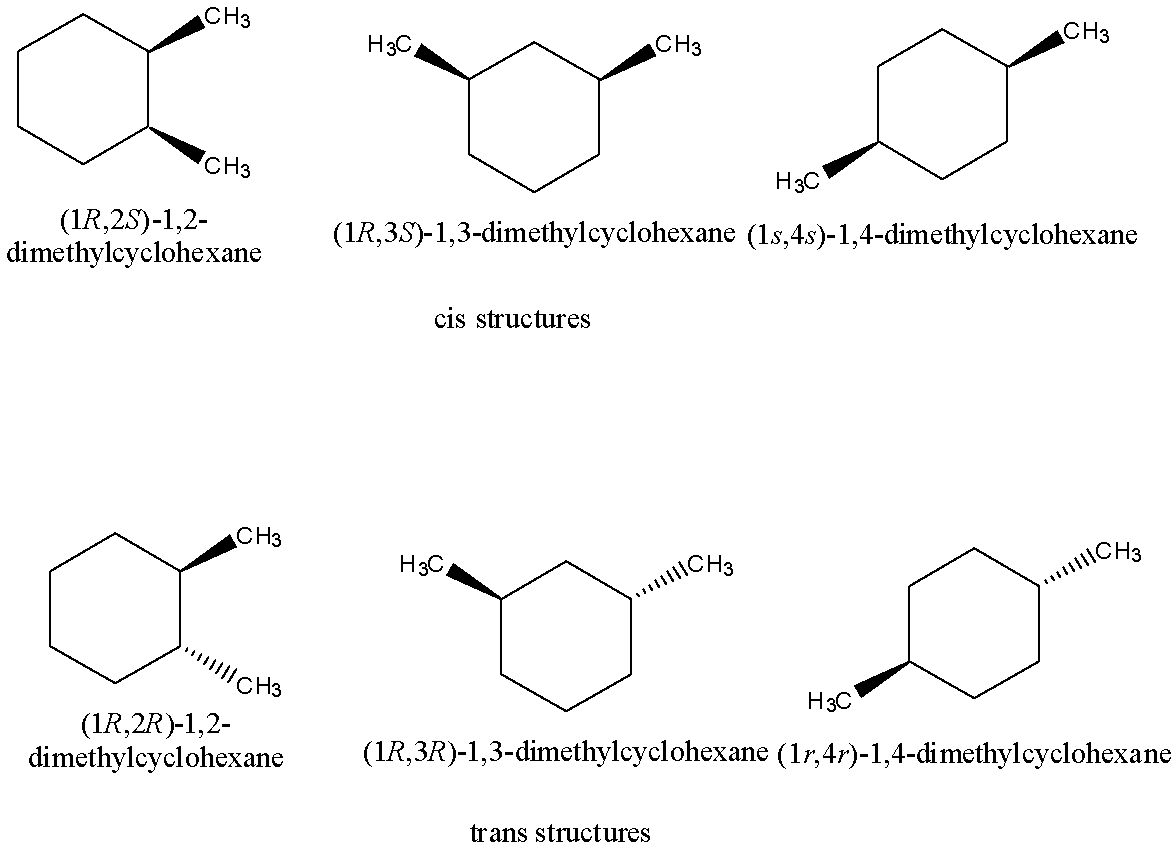
Out of six geometric isomers, three isomers are cis structures and three are trans structures. In geometrical isomers for dimethyl cyclohexane, cis structures are those structures possessing the two methyl groups on the same side of the benzene ring whereas the trans structures are those structures possessing the two methyl groups on the opposite side of the benzene ring.
Therefore, four structural isomers and six geometric isomers are possible for dimethyl cyclohexane.
So, the correct answer is Option B.
Additional Information:
The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon is termed as stereoisomerism. There are six geometrical isomers possible for dimethyl cyclohexane.
Note: It is to be noted that structural isomerism is of different types depending upon the nature of the compounds involved. These are chain isomerism, position isomerism, functional isomerism, metamerism, tautomerism and ring chain isomerism.
Complete step by step answer:
Let’s first discuss structural isomerism in detail. Structural isomerism is shown by the compounds having the same molecular formulae differing in the arrangement of the atoms. There are four possible geometrical isomers for dimethyl cyclohexane.

All the above structural isomers possess the same chemical formula but their structures are different.
Let’s discuss geometrical isomerism in detail. In geometrical isomerism, two types of isomers namely cis and trans present. Geometrical isomerism is a type of stereoisomerism.

Out of six geometric isomers, three isomers are cis structures and three are trans structures. In geometrical isomers for dimethyl cyclohexane, cis structures are those structures possessing the two methyl groups on the same side of the benzene ring whereas the trans structures are those structures possessing the two methyl groups on the opposite side of the benzene ring.
Therefore, four structural isomers and six geometric isomers are possible for dimethyl cyclohexane.
So, the correct answer is Option B.
Additional Information:
The compounds having the same molecular as well as same structural formulae but differing in the relative arrangement of the atoms or groups in space are called stereoisomers and the phenomenon is termed as stereoisomerism. There are six geometrical isomers possible for dimethyl cyclohexane.
Note: It is to be noted that structural isomerism is of different types depending upon the nature of the compounds involved. These are chain isomerism, position isomerism, functional isomerism, metamerism, tautomerism and ring chain isomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




