
How is the strength of the covalent bond related to overlapping of the orbitals?
Answer
523.2k+ views
Hint :When the mutual sharing of electrons takes place between two atoms, the type of bond formed is known as covalent bond. Valence bond theory is one of the most important theories which explains hybridization and strength of covalent bonds formed between the atoms.
Complete Step By Step Answer:
Postulates of Valence bond theory are as follows:
A covalent bond is formed between two atoms when the orbitals overlap each other and an increase in the electron density is observed due to overlapping of orbitals and hence, the stability of the molecule increases.
If there is any unpaired electron present in the orbital of an atom, then the formation of multiple bonds with other atoms takes place.
The paired electrons present in the orbitals do not participate in the bonding.
When the head on head overlapping of orbitals takes place then sigma bonds are formed whereas when the side wise overlapping of orbitals takes place then pi bonds are formed.
The overlapping of orbitals takes place as follows:
s-s overlapping: The s-orbital overlaps to other s-orbital via head-to-head overlapping and formation of sigma bond takes place. The overlapping is represented as follows:
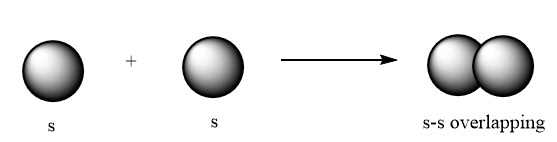
p-p overlapping: The p-orbital overlaps to other p-orbital via head-to-head overlapping and formation of sigma bonds takes place. The overlapping is represented as follows:
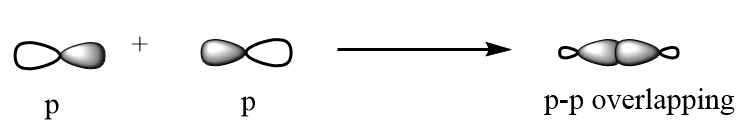
s-p overlapping: The s orbital overlaps to p-orbital via head-to-head overlapping and formation of sigma bond takes place. The overlapping is represented as follows:
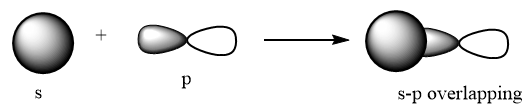
p-p sidewise overlapping: The p-orbital overlaps to other p-orbital in parallel position and formation of pi bond takes place. The overlapping is represented as follows:
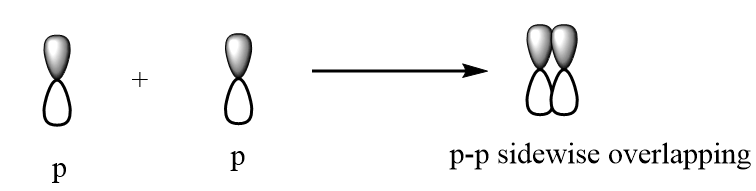
Hence, when two orbitals overlap, the probability of finding electrons depends upon the extent of the overlapping. The greater the overlapping of orbitals, the more energy will be required to break the pair of electrons and therefore on increasing the extent of overlapping, the strength of covalent bond increases.
Note :
It is important to note that the greater the s character in overlapping, greater will be the strength of bond formed because s orbital is closest to the nucleus and thus electrons present in the s orbital will be comparatively more stable. The extent of bond strength follows the order: $s - s > s - p > p - p$.
Complete Step By Step Answer:
Postulates of Valence bond theory are as follows:
A covalent bond is formed between two atoms when the orbitals overlap each other and an increase in the electron density is observed due to overlapping of orbitals and hence, the stability of the molecule increases.
If there is any unpaired electron present in the orbital of an atom, then the formation of multiple bonds with other atoms takes place.
The paired electrons present in the orbitals do not participate in the bonding.
When the head on head overlapping of orbitals takes place then sigma bonds are formed whereas when the side wise overlapping of orbitals takes place then pi bonds are formed.
The overlapping of orbitals takes place as follows:
s-s overlapping: The s-orbital overlaps to other s-orbital via head-to-head overlapping and formation of sigma bond takes place. The overlapping is represented as follows:

p-p overlapping: The p-orbital overlaps to other p-orbital via head-to-head overlapping and formation of sigma bonds takes place. The overlapping is represented as follows:
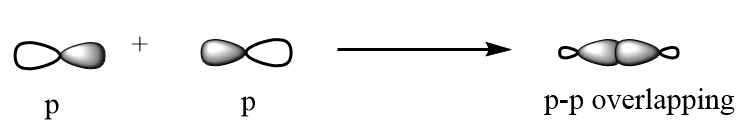
s-p overlapping: The s orbital overlaps to p-orbital via head-to-head overlapping and formation of sigma bond takes place. The overlapping is represented as follows:
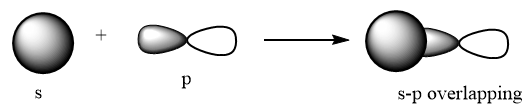
p-p sidewise overlapping: The p-orbital overlaps to other p-orbital in parallel position and formation of pi bond takes place. The overlapping is represented as follows:

Hence, when two orbitals overlap, the probability of finding electrons depends upon the extent of the overlapping. The greater the overlapping of orbitals, the more energy will be required to break the pair of electrons and therefore on increasing the extent of overlapping, the strength of covalent bond increases.
Note :
It is important to note that the greater the s character in overlapping, greater will be the strength of bond formed because s orbital is closest to the nucleus and thus electrons present in the s orbital will be comparatively more stable. The extent of bond strength follows the order: $s - s > s - p > p - p$.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




