
Steric number of the central atom in $ IF_4^ - $ is $ 6 $ . If true, enter $ 1 $ , else enter $ 0 $.
Answer
510.3k+ views
Hint: The compound which is mentioned in the question is an interhalogen compound. Its chemical name is iodine tetra-fluoride. Its existing form has already been given in the question. To find its steric number, we have to know its structure first. After that only we can find its steric number. The complete detailed solution is given in the below section.
Complete answer:
We can draw its structure by using VSEPR (valence shell electron repulsion theory) theory. The structure of $ IF_4^ - $ is shown below.
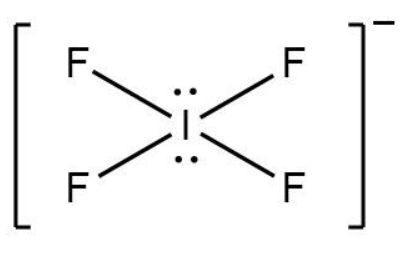
Now the formula to find its steric number is = Number of lone pairs + Number of bonded atoms.
As there are two lone pairs and four bonded atoms, so the steric number $ = 2 + 4 = 6 $ .
Therefore it is true that its steric number is $ 6 $ . The shape of this molecule is square planar and the hybridization of this molecule is $ s{p^3}{d^2} $ . We can find it through the VSEPR theory. As from its hybridization we can clearly see that one s-orbital, three p-orbital, and two d-orbital are mixed to form six hybrid orbitals.
Note:
Iodine forms many inter-halogen compounds such as iodine trifluoride, iodine penta-fluoride, etc. The chemical formula of iodine trifluoride is $ I{F_3} $ and the chemical formula of iodine penta-fluoride is $ I{F_5} $ . The shape of iodine trifluoride is T-shaped based on the VSEPR theory. It contains five electron pairs and out of them two are lone pairs. It is formed by the reaction of fluorine and iodine.
Complete answer:
We can draw its structure by using VSEPR (valence shell electron repulsion theory) theory. The structure of $ IF_4^ - $ is shown below.

Now the formula to find its steric number is = Number of lone pairs + Number of bonded atoms.
As there are two lone pairs and four bonded atoms, so the steric number $ = 2 + 4 = 6 $ .
Therefore it is true that its steric number is $ 6 $ . The shape of this molecule is square planar and the hybridization of this molecule is $ s{p^3}{d^2} $ . We can find it through the VSEPR theory. As from its hybridization we can clearly see that one s-orbital, three p-orbital, and two d-orbital are mixed to form six hybrid orbitals.
Note:
Iodine forms many inter-halogen compounds such as iodine trifluoride, iodine penta-fluoride, etc. The chemical formula of iodine trifluoride is $ I{F_3} $ and the chemical formula of iodine penta-fluoride is $ I{F_5} $ . The shape of iodine trifluoride is T-shaped based on the VSEPR theory. It contains five electron pairs and out of them two are lone pairs. It is formed by the reaction of fluorine and iodine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




