
How many stereoisomers are possible for the following molecule?
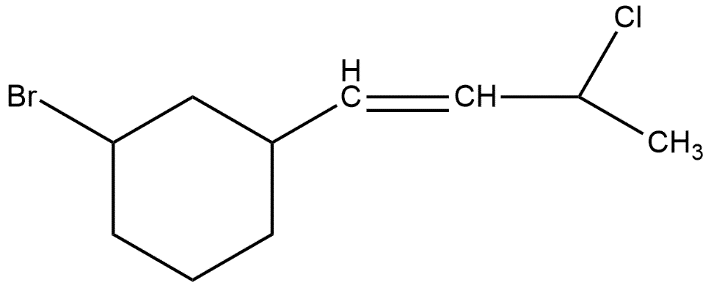
A. 4
B. 8
C. 16
D. 12

Answer
512.7k+ views
Hint: Isomerism is the phenomenon in which two or more compounds have the same molecular formula but different arrangement of atoms. These also differ in physical and chemical properties with each other.
Complete answer:
Stereoisomers are those compounds which have the same molecular formula but different arrangement of atoms in three dimensional space.
In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. This is similar to structural isomers which share the same molecular formula but the bond connections or their order differs.
Hence to find out the number of stereoisomers in the given compound we first have to look about the stereogenic centre atoms present in this compound where the stereo centre atom is that compound which has all the three different groups attached with itself like a chiral carbon atom. In the given compound we can see that 3 stereogenic centres are available.
Number of stereoisomers = \[{{2}^{n}}\]
Where n is number of chiral centers, in this
Number of stereoisomers = \[{{2}^{3}}\]
= 8
Thus, the total number of stereoisomers in the given compound is 8, option B is correct.
Note:
Chiral molecules are those molecules which are not superimposable on their mirror images. Chiral carbon is the carbon which is bonded to different atoms or groups also known by asymmetric carbon.
Complete answer:
Stereoisomers are those compounds which have the same molecular formula but different arrangement of atoms in three dimensional space.
In stereochemistry, stereoisomerism or spatial isomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms but differ in the three-dimensional orientations of their atoms in space. This is similar to structural isomers which share the same molecular formula but the bond connections or their order differs.
Hence to find out the number of stereoisomers in the given compound we first have to look about the stereogenic centre atoms present in this compound where the stereo centre atom is that compound which has all the three different groups attached with itself like a chiral carbon atom. In the given compound we can see that 3 stereogenic centres are available.
Number of stereoisomers = \[{{2}^{n}}\]
Where n is number of chiral centers, in this
Number of stereoisomers = \[{{2}^{3}}\]
= 8
Thus, the total number of stereoisomers in the given compound is 8, option B is correct.
Note:
Chiral molecules are those molecules which are not superimposable on their mirror images. Chiral carbon is the carbon which is bonded to different atoms or groups also known by asymmetric carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




