
How many stereoisomers and chiral carbons are there on a \[1,3,5-trimethyl\text{ }cyclohexane\] molecule?
Answer
535.5k+ views
Hint: In order to understand chirality and stereoisomers, one must understand the concept of spatial arrangement. Spatial arrangement of atoms means how different atomic particles and molecules are situated in the space around the organic compound, namely its carbon chain. In this sense, the spatial arrangement of an organic molecule is different if an atom is shifted in any three-dimensional direction by even one degree.
Complete step by step solution:
Chirality means 'mirror-image, non-superimposable molecules', and we can say that a molecule is chiral if its mirror image (it must have one) is not the same as itself.
A carbon atom that is bonded to four different atoms or groups loses all symmetry, and is often referred to as an asymmetric carbon. The configuration of such a molecular unit is called a chiral, and the structure may exist in either a right-handed configuration or a left-handed configuration (one the mirror image of the other). This type of configurationally stereoisomers is termed enantiomorphism, and the non-identical, mirror-image pair of stereoisomers that result are called enantiomers
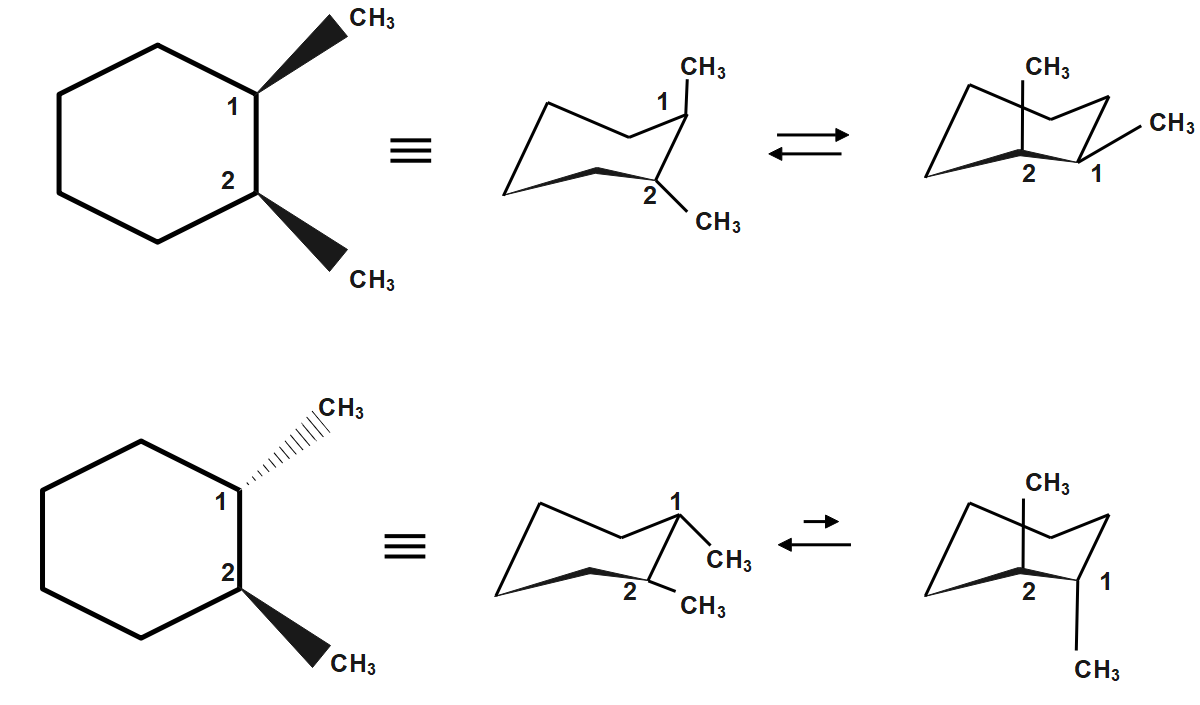
In hexane all the C-atoms are symmetric, so no carbon atom is chiral. In n-butane also all C-atom present are symmetric .hence it is achiral In methane, all groups attached are the same. Stereogenic elements may be chiral or achiral. An asymmetric carbon is often a chiral stereogenic center, since interchanging any two substituent groups converts one enantiomer to the other. Alkenes having two different groups on each double bond carbon constitute an achiral stereogenic element, since interchanging substituents at one of the carbons changes the cis/trans configuration. Hence it is also achiral.
In \[1,3,5-trimethyl\text{ }cyclohexane\] there are two chiral centers making the compound asymmetric. Hence \[1,3,5-trimethyl\text{ }cyclohexane\] is a chiral compound.
Note: A useful first step in examining structural formulas to determine whether stereoisomers may exist is to identify all stereogenic elements. A stereogenic element is a center, axis or plane that is a focus of stereoisomerism, such that an interchange of two groups attached to this feature leads to a stereoisomer.
Complete step by step solution:
Chirality means 'mirror-image, non-superimposable molecules', and we can say that a molecule is chiral if its mirror image (it must have one) is not the same as itself.
A carbon atom that is bonded to four different atoms or groups loses all symmetry, and is often referred to as an asymmetric carbon. The configuration of such a molecular unit is called a chiral, and the structure may exist in either a right-handed configuration or a left-handed configuration (one the mirror image of the other). This type of configurationally stereoisomers is termed enantiomorphism, and the non-identical, mirror-image pair of stereoisomers that result are called enantiomers

In hexane all the C-atoms are symmetric, so no carbon atom is chiral. In n-butane also all C-atom present are symmetric .hence it is achiral In methane, all groups attached are the same. Stereogenic elements may be chiral or achiral. An asymmetric carbon is often a chiral stereogenic center, since interchanging any two substituent groups converts one enantiomer to the other. Alkenes having two different groups on each double bond carbon constitute an achiral stereogenic element, since interchanging substituents at one of the carbons changes the cis/trans configuration. Hence it is also achiral.
In \[1,3,5-trimethyl\text{ }cyclohexane\] there are two chiral centers making the compound asymmetric. Hence \[1,3,5-trimethyl\text{ }cyclohexane\] is a chiral compound.
Note: A useful first step in examining structural formulas to determine whether stereoisomers may exist is to identify all stereogenic elements. A stereogenic element is a center, axis or plane that is a focus of stereoisomerism, such that an interchange of two groups attached to this feature leads to a stereoisomer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




