
Statement: In $\,{N_2}{O_{3\,}}\,$, the central $\,O\,$ atom is $\,s{p^3}\,$ hybridized and each $\,N\,$ atom is $\,s{p^2}\,$ hybridized.
If the given statement is true enter $\,1\,$ else $\,0\,$.
Answer
580.8k+ views
Hint: $\,s{p^3}$ hybridization involves the intermixing of one $\,s\,$orbital and three $\,p\,$orbitals. Whereas $\,s{p^2}\,$ hybridization involves one $\,s\,$ orbital and two $\,p\,$ orbitals.
Complete step by step answer:
Let’s first learn about hybridization and its mechanisms. The concept of hybridization of atomic orbitals was introduced by Linus Pauling in an attempt to explain the geometries, nature of bonds, shapes, and bond angles of polyatomic molecules. In simple terms, we can describe hybridization as follows;
Hybridization is defined as the process of intermixing of various atomic orbitals of slightly different energies in the same atom with the redistribution of their energies to yield a new set of the same number of orbitals of equivalent energies and shapes.
Mathematically, a hybrid orbital is a linear combination of atomic orbitals of the same atom.
There are different types of hybridization-based on the orbitals participating in it. They include $\,s{p^3}\,,\,s{p^2},\,sp\,,\,s{p^3}d\,,\,s{p^3}{d^2}\,,\,s{p^3}{d^3}\,$ etc.
Now let’s come to the question. In this question, the compound given is dinitrogen trioxide $\,({N_2}{O_5})\,$ .
In this compound, we can see that the central oxygen atom contains two lone pairs of electrons and two bond pairs of electrons. These lone pairs of electrons take part in hybridization and one $\,s\,$ orbital and three $\,p\,$ orbitals mix among themselves with the redistribution of energy to yield four new $\,s{p^3}\,$ hybridized orbitals.
Now, in the case of nitrogen atoms, they contain two bond pairs of electrons and one lone pair. So these lone pairs take part in hybridization by the intermixing of one $\,s\,$orbital and two $\,p\,$ orbitals to form three new $\,s{p^2}\,$ hybridized orbitals.
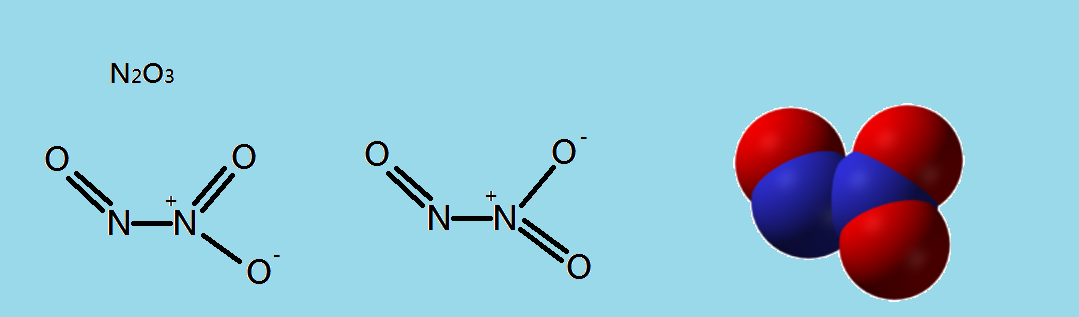
From the above explanations, it is clear that the statement mentioned in the question is true so that we can enter $\,1\,$ .
Note:
In $\,s{p^3}\,$ hybridization, the orbitals are oriented in a tetrahedral manner. Whereas in $\,s{p^2}\,$ hybridization, the orbitals are oriented in a trigonal planar manner. The hybrid orbitals are oriented in space in preferred directions such that there is the least repulsion between electron pairs and there is a stable arrangement.
Complete step by step answer:
Let’s first learn about hybridization and its mechanisms. The concept of hybridization of atomic orbitals was introduced by Linus Pauling in an attempt to explain the geometries, nature of bonds, shapes, and bond angles of polyatomic molecules. In simple terms, we can describe hybridization as follows;
Hybridization is defined as the process of intermixing of various atomic orbitals of slightly different energies in the same atom with the redistribution of their energies to yield a new set of the same number of orbitals of equivalent energies and shapes.
Mathematically, a hybrid orbital is a linear combination of atomic orbitals of the same atom.
There are different types of hybridization-based on the orbitals participating in it. They include $\,s{p^3}\,,\,s{p^2},\,sp\,,\,s{p^3}d\,,\,s{p^3}{d^2}\,,\,s{p^3}{d^3}\,$ etc.
Now let’s come to the question. In this question, the compound given is dinitrogen trioxide $\,({N_2}{O_5})\,$ .
In this compound, we can see that the central oxygen atom contains two lone pairs of electrons and two bond pairs of electrons. These lone pairs of electrons take part in hybridization and one $\,s\,$ orbital and three $\,p\,$ orbitals mix among themselves with the redistribution of energy to yield four new $\,s{p^3}\,$ hybridized orbitals.
Now, in the case of nitrogen atoms, they contain two bond pairs of electrons and one lone pair. So these lone pairs take part in hybridization by the intermixing of one $\,s\,$orbital and two $\,p\,$ orbitals to form three new $\,s{p^2}\,$ hybridized orbitals.

From the above explanations, it is clear that the statement mentioned in the question is true so that we can enter $\,1\,$ .
Note:
In $\,s{p^3}\,$ hybridization, the orbitals are oriented in a tetrahedral manner. Whereas in $\,s{p^2}\,$ hybridization, the orbitals are oriented in a trigonal planar manner. The hybrid orbitals are oriented in space in preferred directions such that there is the least repulsion between electron pairs and there is a stable arrangement.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




