
Statement: Aluminium chloride $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ is a Lewis acid because it can donate the electron.
If a given statement is true enter 1 if false enter 0.
Answer
572.1k+ views
Hint: Lewis suggested the acid-base concept. According to which the Lewis base is a chemical species that can donate the electron pair and Lewis base is a chemical species that can easily accept the electron pair. $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ has an empty orbital and accommodates the electron pair in an empty orbital.
Complete answer:
Lewis acid is an ion or a molecule that accepts the nonbonding valence electrons in its empty orbital. Lewis base is an ion or molecule which can donate an electron pair.
Aluminium trichloride $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ is an electron-deficient species.it has three electrons in the valence shell. The electronic configuration of aluminium is $\text{ Al = }\left[ \text{Ne} \right]\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{1}}}\text{3}{{\text{d}}^{\text{0 }}}$
In $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ aluminium loses its three valence shell electrons. Two electrons from the 3s orbitals and one electron from the 3p orbital. When bonded with chlorine $\text{ A}{{\text{l}}^{\text{3+ }}}$ has empty 3s, 3p, and 3d orbitals. These empty orbitals are used to accommodate the nonbonding pair of the electron which are donated by the Lewis base.
Let’s consider a reaction of $\text{ A}{{\text{l}}^{\text{3+ }}}$ the water molecule. The $\text{ A}{{\text{l}}^{\text{3+ }}}$ ion is an electron-deficient species and bonds three chloride molecules. The bonding between the aluminium and chloride is as shown below,
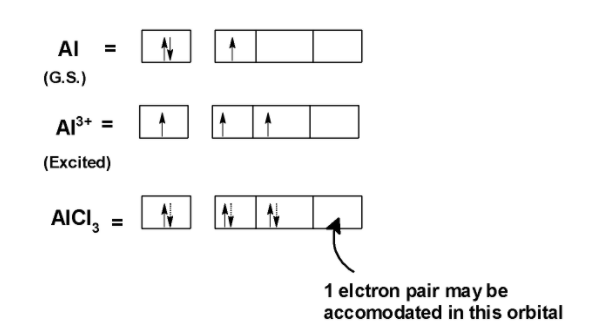
One orbital of the $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ is empty. This orbit can accommodate the nonbonding electron pair of electrons. Thus $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ acts as a Lewis acid because it can accept an electron.
Thus the given statement is false.
Hence, ‘0’ is the correct answer.
Note: Lewis acid is used as a catalyst in Friedel craft reaction. $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ accepts the lone pair from chloride ion and form the $\text{ AlCl}_{4}^{-}\text{ }$ ion. This forms a carbonium ion which further acts as a strong Lewis acid. Thus Friedel craft acylation and alkylation reactions are facilitated by $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ the catalyst.
Complete answer:
Lewis acid is an ion or a molecule that accepts the nonbonding valence electrons in its empty orbital. Lewis base is an ion or molecule which can donate an electron pair.
Aluminium trichloride $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ is an electron-deficient species.it has three electrons in the valence shell. The electronic configuration of aluminium is $\text{ Al = }\left[ \text{Ne} \right]\text{3}{{\text{s}}^{\text{2}}}\text{3}{{\text{p}}^{\text{1}}}\text{3}{{\text{d}}^{\text{0 }}}$
In $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ aluminium loses its three valence shell electrons. Two electrons from the 3s orbitals and one electron from the 3p orbital. When bonded with chlorine $\text{ A}{{\text{l}}^{\text{3+ }}}$ has empty 3s, 3p, and 3d orbitals. These empty orbitals are used to accommodate the nonbonding pair of the electron which are donated by the Lewis base.
Let’s consider a reaction of $\text{ A}{{\text{l}}^{\text{3+ }}}$ the water molecule. The $\text{ A}{{\text{l}}^{\text{3+ }}}$ ion is an electron-deficient species and bonds three chloride molecules. The bonding between the aluminium and chloride is as shown below,

One orbital of the $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ is empty. This orbit can accommodate the nonbonding electron pair of electrons. Thus $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ acts as a Lewis acid because it can accept an electron.
Thus the given statement is false.
Hence, ‘0’ is the correct answer.
Note: Lewis acid is used as a catalyst in Friedel craft reaction. $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ accepts the lone pair from chloride ion and form the $\text{ AlCl}_{4}^{-}\text{ }$ ion. This forms a carbonium ion which further acts as a strong Lewis acid. Thus Friedel craft acylation and alkylation reactions are facilitated by $\text{ AlC}{{\text{l}}_{\text{3}}}\text{ }$ the catalyst.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




