
Statement 1: The molecule $S{{O}_{2}}$ has a net dipole.
Statement 2: Oxygen has a higher electronegativity than sulphur.
(A) Both statement 1 and statement 2 are correct and statement 2 is the correct explanation of the statement 1.
(B) Both statement 1 and statement 2 are correct, but statement 2 is NOT the correct explanation of statement 1.
(C) Statement 1 is correct, but statement 2 is not correct.
(D) Statement 1 is not correct, but statement 2 is correct.
Answer
584.4k+ views
Hint: The dipole moment in any compound is directed towards the more electronegative atom of the compound. The lone pair-lone pair repulsion is much greater than the lone pair-bond pair and bond pair-bond pair repulsion.
Complete step by step answer:
$S{{O}_{2}}$ has a net dipole moment. This is because there is an appreciable difference of electronegativity between Sulphur and Oxygen atoms. Since Oxygen is more electronegative, the electrons on the sulphur atoms are attracted toward the oxygen atom, creating a net dipole moment. Also $S{{O}_{2}}$ has two lone pairs on the central atom, which cause lone pair-lone pair repulsion, which bents the structure of $S{{O}_{2}}$, therefore, the dipole moment have a non zero value.
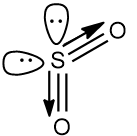
So, the correct answer is “Option A”.
Note: Dipole moment is any compound that arises when there is separation of charges. Dipole moment can arise in ionic compounds and covalent compounds. Dipole moments occur due to the difference in the electronegativity of the two bonded atoms. A dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule.
Complete step by step answer:
$S{{O}_{2}}$ has a net dipole moment. This is because there is an appreciable difference of electronegativity between Sulphur and Oxygen atoms. Since Oxygen is more electronegative, the electrons on the sulphur atoms are attracted toward the oxygen atom, creating a net dipole moment. Also $S{{O}_{2}}$ has two lone pairs on the central atom, which cause lone pair-lone pair repulsion, which bents the structure of $S{{O}_{2}}$, therefore, the dipole moment have a non zero value.

So, the correct answer is “Option A”.
Note: Dipole moment is any compound that arises when there is separation of charges. Dipole moment can arise in ionic compounds and covalent compounds. Dipole moments occur due to the difference in the electronegativity of the two bonded atoms. A dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




