
Statement 1: Reduction of but-2-yne by Na/liq. $N{{H}_{3}}$ gives trans but-2-ene.
Statement 2: It is an example of anti-addition.
A. Statement-1 is true, statement-2 is true and statement-2 is the correct explanation for the statement-1.
B. Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for the statement-1.
C. Statement-1 is true, statement-2 is false
D. Statement-1 is false, statement-2 is true
Answer
533.4k+ views
Hint: The process of addition of electrons or addition of hydrogens is called reduction. The structure of but-2-yne is as follows.
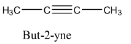
There is a triple bond at the center of the molecule.
Complete step by step answer:
- In the question it is asked to find the correct statements among the given two statements.
- Generally the compound which contains double or rippled bonds undergoes additional reactions and forms saturated compounds as the products.
- Generally reduction reactions are going to carry out the presence of hydrogen in the presence of Lindlar catalysts.
- In the presence of the Lindlar catalyst the product formed by the but-2-yne is cis -2-butene.
- But in the question it is given that but-2-yne is going to react with liquid ammonia in the presence of sodium.
- The chemical reaction of but-2-yne with hydrogen Lindlar catalyst and the sodium liquid ammonia is as follows.
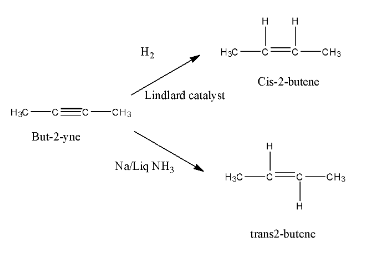
- Therefore by observing the given statements
Statement 1: Reduction of but-2-yne by Na/liq. $N{{H}_{3}}$ gives trans but-2-ene.
Statement 2: It is an example of anti-addition.
Both the statements are correct and statement 2 is a right explanation form statement 1.
Therefore the correct option is option A.
Note: In the presence of sodium and liquid ammonia anti addition takes place and leads to the formation of trans-2-butene as the product. Linlard catalyst favors the formation of the cis but-2-ene as the product.
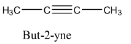
There is a triple bond at the center of the molecule.
Complete step by step answer:
- In the question it is asked to find the correct statements among the given two statements.
- Generally the compound which contains double or rippled bonds undergoes additional reactions and forms saturated compounds as the products.
- Generally reduction reactions are going to carry out the presence of hydrogen in the presence of Lindlar catalysts.
- In the presence of the Lindlar catalyst the product formed by the but-2-yne is cis -2-butene.
- But in the question it is given that but-2-yne is going to react with liquid ammonia in the presence of sodium.
- The chemical reaction of but-2-yne with hydrogen Lindlar catalyst and the sodium liquid ammonia is as follows.

- Therefore by observing the given statements
Statement 1: Reduction of but-2-yne by Na/liq. $N{{H}_{3}}$ gives trans but-2-ene.
Statement 2: It is an example of anti-addition.
Both the statements are correct and statement 2 is a right explanation form statement 1.
Therefore the correct option is option A.
Note: In the presence of sodium and liquid ammonia anti addition takes place and leads to the formation of trans-2-butene as the product. Linlard catalyst favors the formation of the cis but-2-ene as the product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




