
State whether following statement is True or False:
\[SnC{{l}_{2}}\] is a non-linear molecule.
Answer
591.6k+ views
Hint: The shape of the molecules is going to identify easily by using VSEPR theory. By using VSEPR theory (Valence Shell Electron Pair Repulsion theory) we can find the structure and hybridization of the molecules.
Complete answer:
As per VSEPR theory there is a formula to calculate the number of electrons in the molecules.
\[\text{Number of electrons = }\dfrac{1}{2}[V+N-C+A]\]
Here, V = number of valence electrons present on the central atom
N = number of monovalent atoms bonded to the central atom
C = charge of the cation
A = charge of the anion
By using the above formula we can calculate the number of electrons in\[SnC{{l}_{2}}\].
We know that for \[SnC{{l}_{2}}\], V =4 , N =2, C = 0 and A = 0
\[\begin{align}
& \text{Number of electrons = }\dfrac{1}{2}[V+N-C+A] \\
& \text{ = }\dfrac{1}{2}[4+2-0+0] \\
& \text{ = 3} \\
\end{align}\]
The number of electrons in \[SnC{{l}_{2}}\] is 3. That means the hybridization of \[SnC{{l}_{2}}\] is \[s{{p}^{2}}\]and the geometry of the molecule according to VSEPR is trigonal planar. There are two bond pairs, and the third position will be occupied by a lone pair of electrons. Then the shape of the\[SnC{{l}_{2}}\] is bent or non-linear with a bond length less than\[{{120}^{o}}\].
We can draw the structure of the\[SnC{{l}_{2}}\] from the above data as follows.
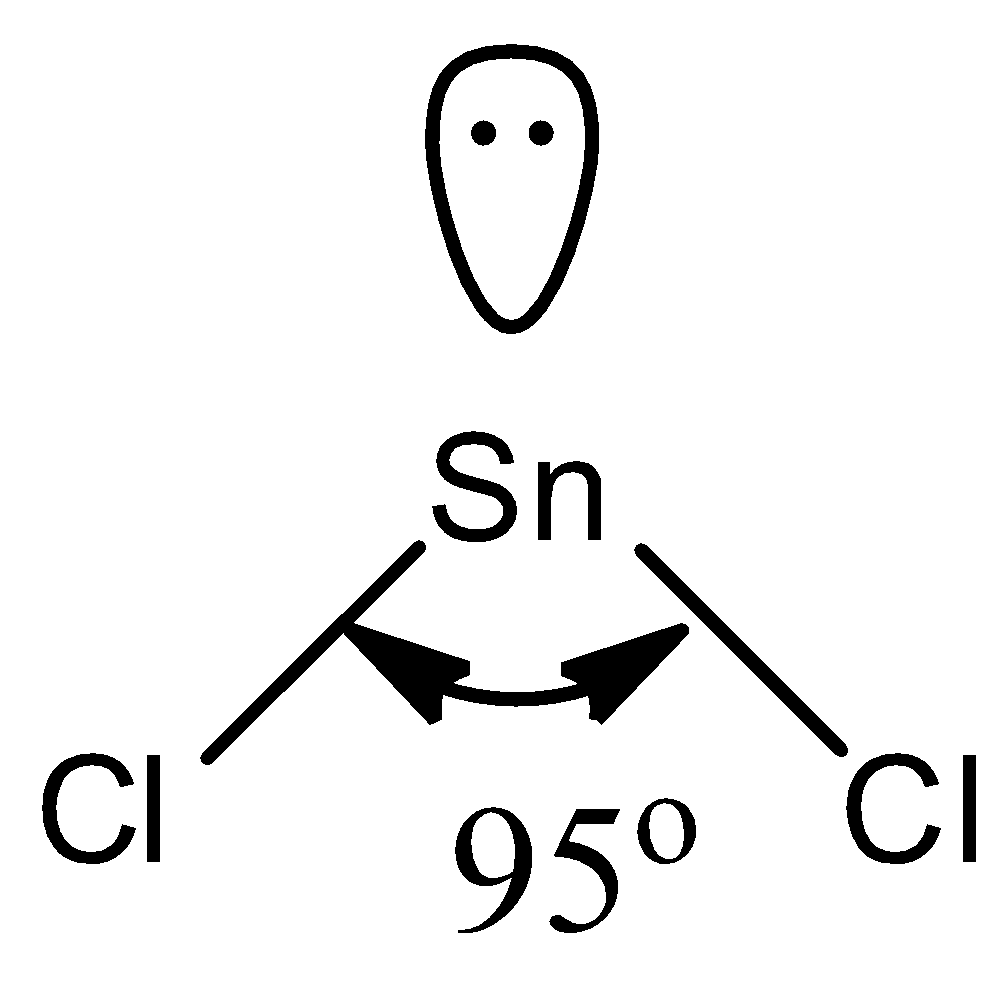
So, the statement is true, \[SnC{{l}_{2}}\] is a non-linear molecule.
Note:
Due to repulsion between the bond pair and lone pair of electrons the shape of the molecule is going to change from linear to bent angular symmetry or V-shaped structure. There are two bond pairs and one lone pair of electrons is present in \[SnC{{l}_{2}}\].
Complete answer:
As per VSEPR theory there is a formula to calculate the number of electrons in the molecules.
\[\text{Number of electrons = }\dfrac{1}{2}[V+N-C+A]\]
Here, V = number of valence electrons present on the central atom
N = number of monovalent atoms bonded to the central atom
C = charge of the cation
A = charge of the anion
By using the above formula we can calculate the number of electrons in\[SnC{{l}_{2}}\].
We know that for \[SnC{{l}_{2}}\], V =4 , N =2, C = 0 and A = 0
\[\begin{align}
& \text{Number of electrons = }\dfrac{1}{2}[V+N-C+A] \\
& \text{ = }\dfrac{1}{2}[4+2-0+0] \\
& \text{ = 3} \\
\end{align}\]
The number of electrons in \[SnC{{l}_{2}}\] is 3. That means the hybridization of \[SnC{{l}_{2}}\] is \[s{{p}^{2}}\]and the geometry of the molecule according to VSEPR is trigonal planar. There are two bond pairs, and the third position will be occupied by a lone pair of electrons. Then the shape of the\[SnC{{l}_{2}}\] is bent or non-linear with a bond length less than\[{{120}^{o}}\].
We can draw the structure of the\[SnC{{l}_{2}}\] from the above data as follows.

So, the statement is true, \[SnC{{l}_{2}}\] is a non-linear molecule.
Note:
Due to repulsion between the bond pair and lone pair of electrons the shape of the molecule is going to change from linear to bent angular symmetry or V-shaped structure. There are two bond pairs and one lone pair of electrons is present in \[SnC{{l}_{2}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




