
State true or false
${{Xe}}{{{F}}_{{4}}}$ molecule is square planar in shape
A.True
B.False
Answer
578.7k+ views
Hint: Shape of a molecule can also be mentioned as the geometry of a molecule. Geometry is the arrangement of the atoms and the bonds in a molecule. The molecular shapes are linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral. This can be identified based on the hybridization of the molecule. There are various theories to determine the shape of a molecule. VSEPR is one theory.
Complete step by step answer:
The shape of a molecule can be found out through VSEPR Theory
The shape of the molecule can be derived from the Lewis dot structures.
According to the VSEPR theory, a molecule will be in a geometry that will minimize the repulsion between the electrons in the valence shell of the central atom. We know that electrons are negatively charged and there will be repulsion.
In ${{Xe}}{{{F}}_{{4}}}$, the central atom Xe is a noble gas and has eight electrons in its valence shell and each Fluorine has seven electrons in its valence shell. So ${{Xe}}{{{F}}_{{4}}}$ has a total of ${{8 + 7(4) = 36}}$ electrons in its valence shell.
So let us arrange 36 electrons around the molecule. There is a 4 F atom which can be bonded to the Xe.
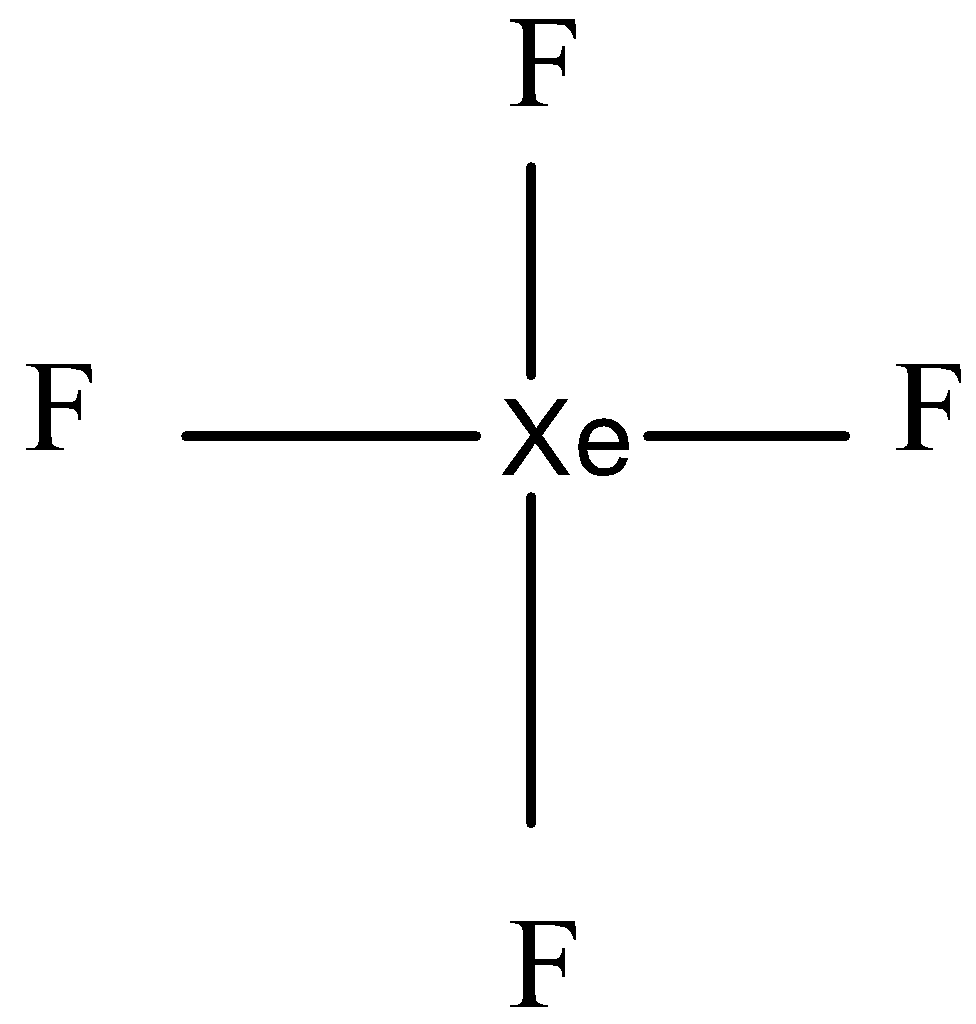
Let us now put all the 36 electrons around the molecule. 6 electrons can be arranged around each F atom because 1 electron is bonded to Xe.
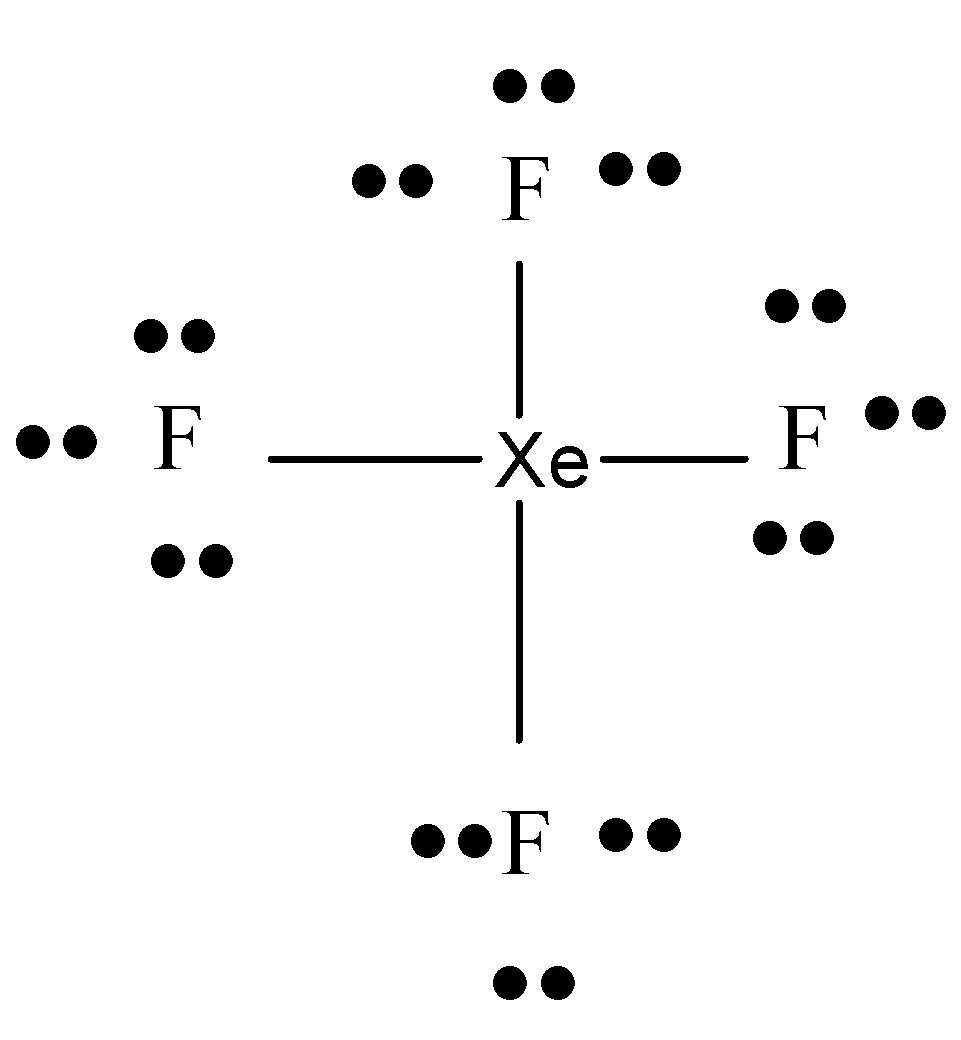
Now let us count the total number of electrons. There are 24 electrons in total on the fluorine atoms and 4 bonds (8 electrons). So 32 electrons out of the 36 are shared.
There are 4 more electrons and it is given to Xe. So it has 2 lone pairs and 4 bond pairs.
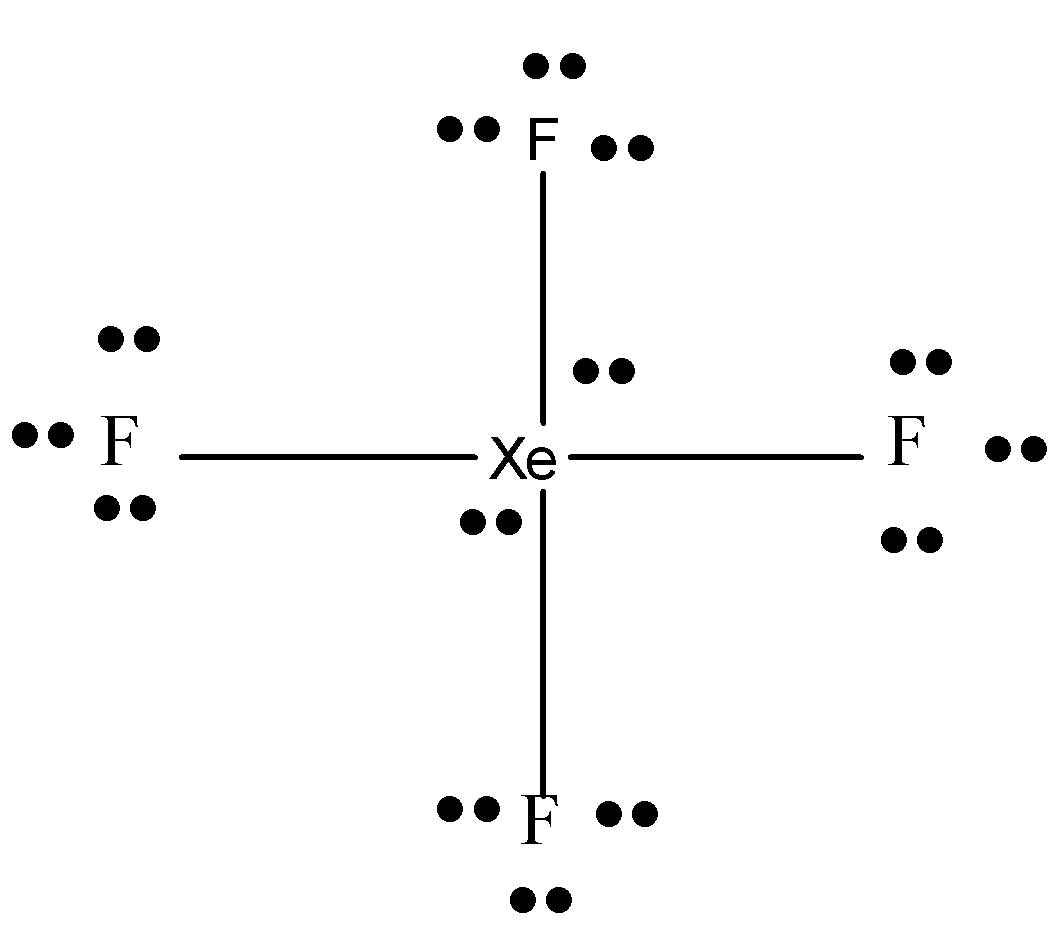
So we can say that this molecule is ${{A}}{{{X}}_{{4}}}{{{E}}_{{2}}}$ where the central atom has 4 bond pairs and 2 lone pairs of electrons.
A molecule with 4 bond pairs are 2 lone pairs arranged as a square planar molecule.
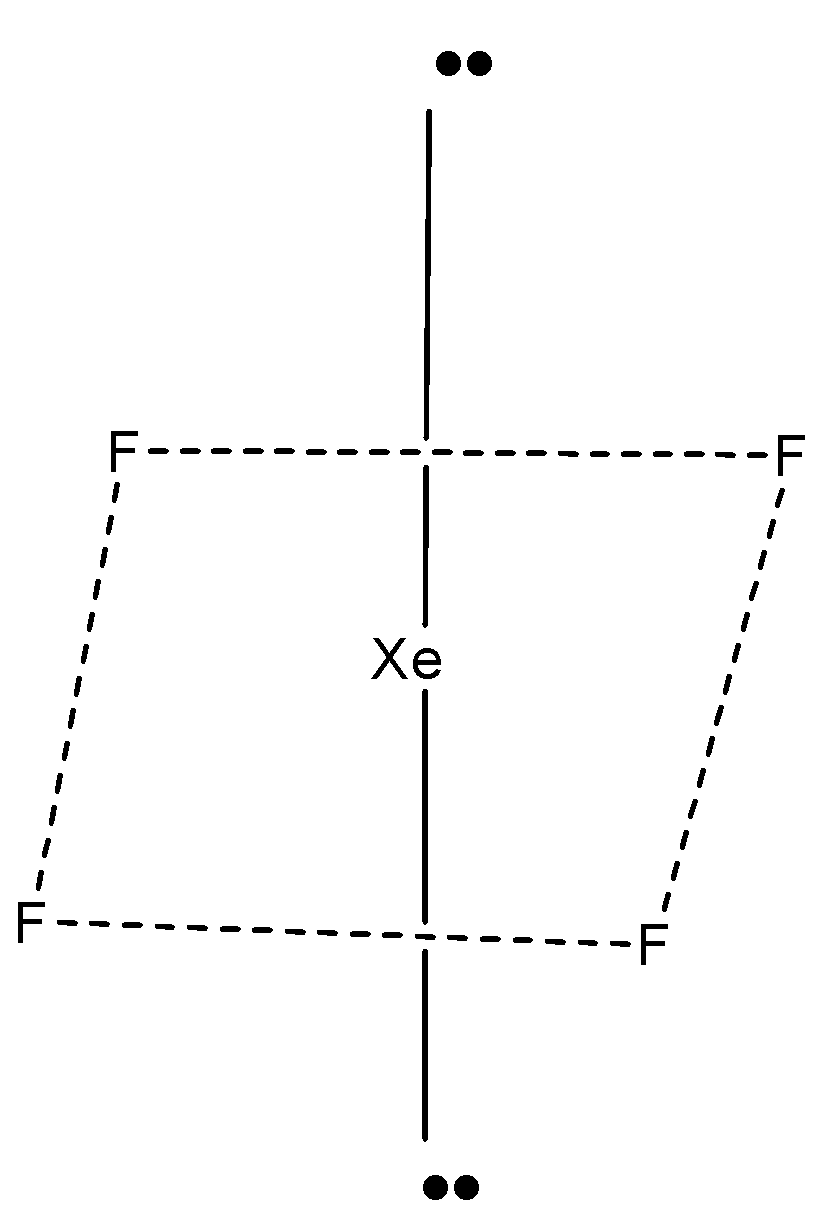
This shape is called the square planar. In this, the electron pair is arranged such that there is minimum repulsion.
Therefore the statement is true.
The correct option is (A).
Note:
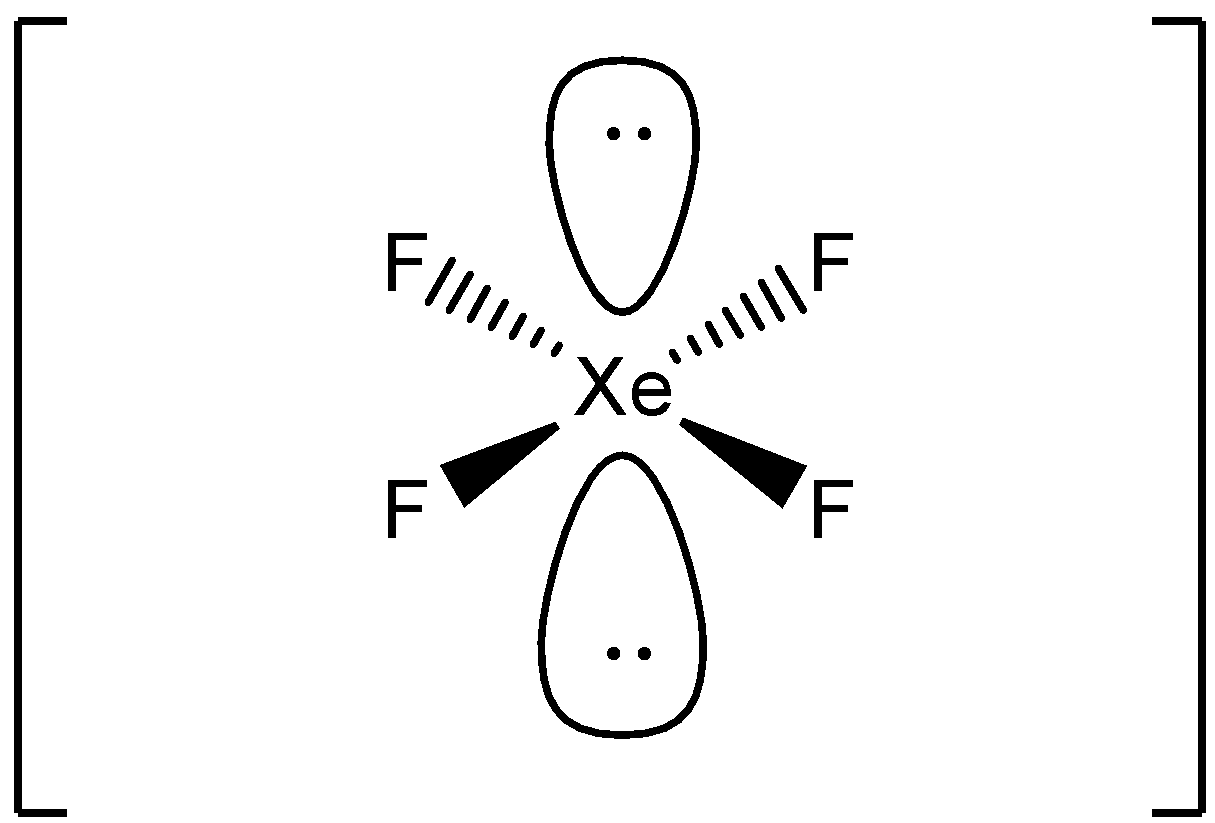
Due to its \[sp{}^3d{}^2\] hybridization, it results in the octahedral geometry. But according to the VSEPR rule, \[XeF{}_4\] shows square planar geometry. In this molecule, Xenon has an expanded octet. According to the VSEPR theory, the repulsion follows the order. ${{lone pair - lone pair > lone pair - bond pair > bond pair - bond pair}}$. So the lone pairs are repelled more. So in this molecule, lone pairs are arranged opposite to minimize the repulsion. ${{Xe}}{{{F}}_{{4}}}$ is a nonpolar molecule because the Fluorine atoms are pulling equally in all directions and it gets canceled.
Complete step by step answer:
The shape of a molecule can be found out through VSEPR Theory
The shape of the molecule can be derived from the Lewis dot structures.
According to the VSEPR theory, a molecule will be in a geometry that will minimize the repulsion between the electrons in the valence shell of the central atom. We know that electrons are negatively charged and there will be repulsion.
In ${{Xe}}{{{F}}_{{4}}}$, the central atom Xe is a noble gas and has eight electrons in its valence shell and each Fluorine has seven electrons in its valence shell. So ${{Xe}}{{{F}}_{{4}}}$ has a total of ${{8 + 7(4) = 36}}$ electrons in its valence shell.
So let us arrange 36 electrons around the molecule. There is a 4 F atom which can be bonded to the Xe.
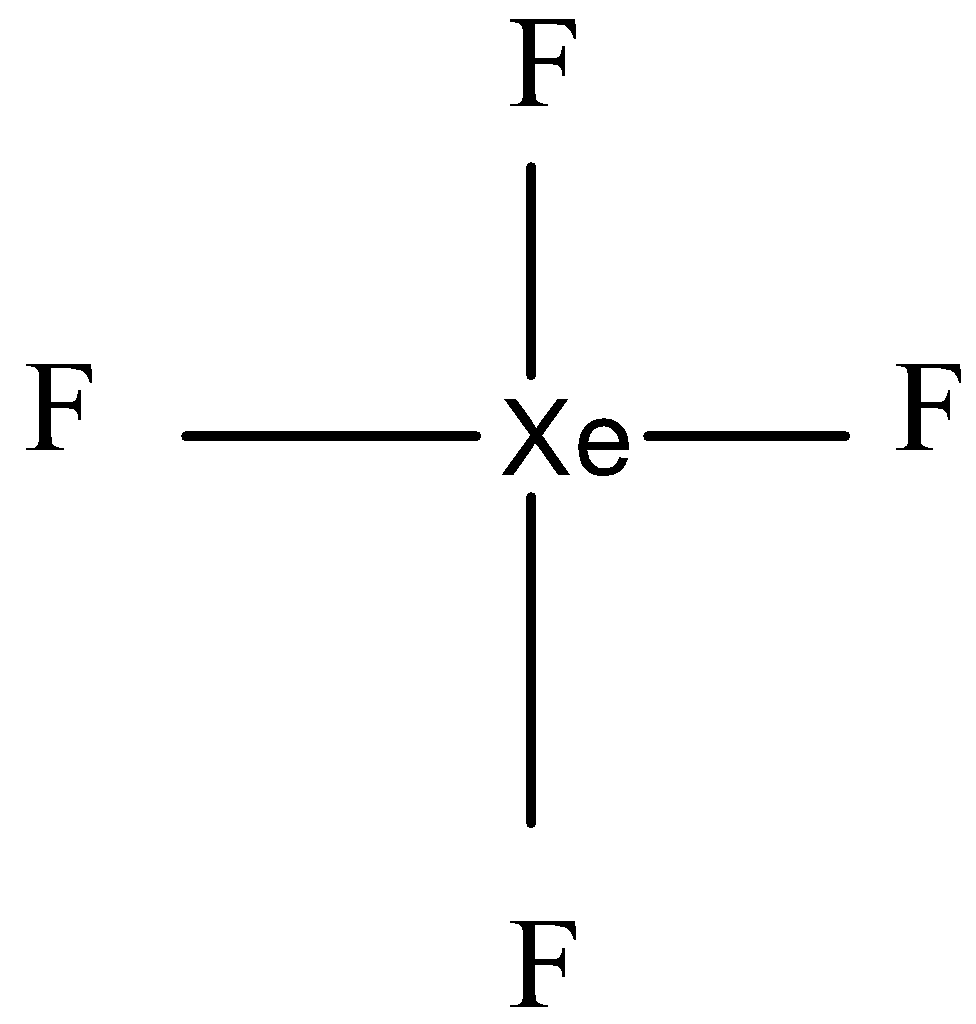
Let us now put all the 36 electrons around the molecule. 6 electrons can be arranged around each F atom because 1 electron is bonded to Xe.

Now let us count the total number of electrons. There are 24 electrons in total on the fluorine atoms and 4 bonds (8 electrons). So 32 electrons out of the 36 are shared.
There are 4 more electrons and it is given to Xe. So it has 2 lone pairs and 4 bond pairs.

So we can say that this molecule is ${{A}}{{{X}}_{{4}}}{{{E}}_{{2}}}$ where the central atom has 4 bond pairs and 2 lone pairs of electrons.
A molecule with 4 bond pairs are 2 lone pairs arranged as a square planar molecule.

This shape is called the square planar. In this, the electron pair is arranged such that there is minimum repulsion.
Therefore the statement is true.
The correct option is (A).
Note:

Due to its \[sp{}^3d{}^2\] hybridization, it results in the octahedral geometry. But according to the VSEPR rule, \[XeF{}_4\] shows square planar geometry. In this molecule, Xenon has an expanded octet. According to the VSEPR theory, the repulsion follows the order. ${{lone pair - lone pair > lone pair - bond pair > bond pair - bond pair}}$. So the lone pairs are repelled more. So in this molecule, lone pairs are arranged opposite to minimize the repulsion. ${{Xe}}{{{F}}_{{4}}}$ is a nonpolar molecule because the Fluorine atoms are pulling equally in all directions and it gets canceled.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




