
State True or False
The $C{H_2}C{l_2}$ molecule may be polar or nonpolar depending on its geometry
A. True
B. False
Answer
558.9k+ views
Hint: We must know that the polarity of the molecule depends on the dipole moment. The dipole moment depends on the direction of the dipole. The one molecule is said to be polar, compulsory it having net dipole moment. The dipole moments in the bonding atoms sometimes cancel each other. Because the direction of the dipole will be opposite.
Complete step by step answer:
We must remember that the molecule $C{H_2}C{l_2}$ is called methylene dichloride. It is popularly called dichloromethane. Simply called DCM. It is one of the highly polar solvent in organic chemistry.
As we know that in $C{H_2}C{l_2}$ having four dipole bonds. The two dipole bonds are between $C - H$ . But these two dipole moments are present in opposite cancel each other. Other two dipole bonds are between $C - Cl$ . These two dipole bonds are given a net dipole moment of the molecule. These two $C - Cl$ dipole bonds do not cancel each other because they are present in the same direction in the molecule.
The above discussion is easily understand by its molecule diagram is given below,
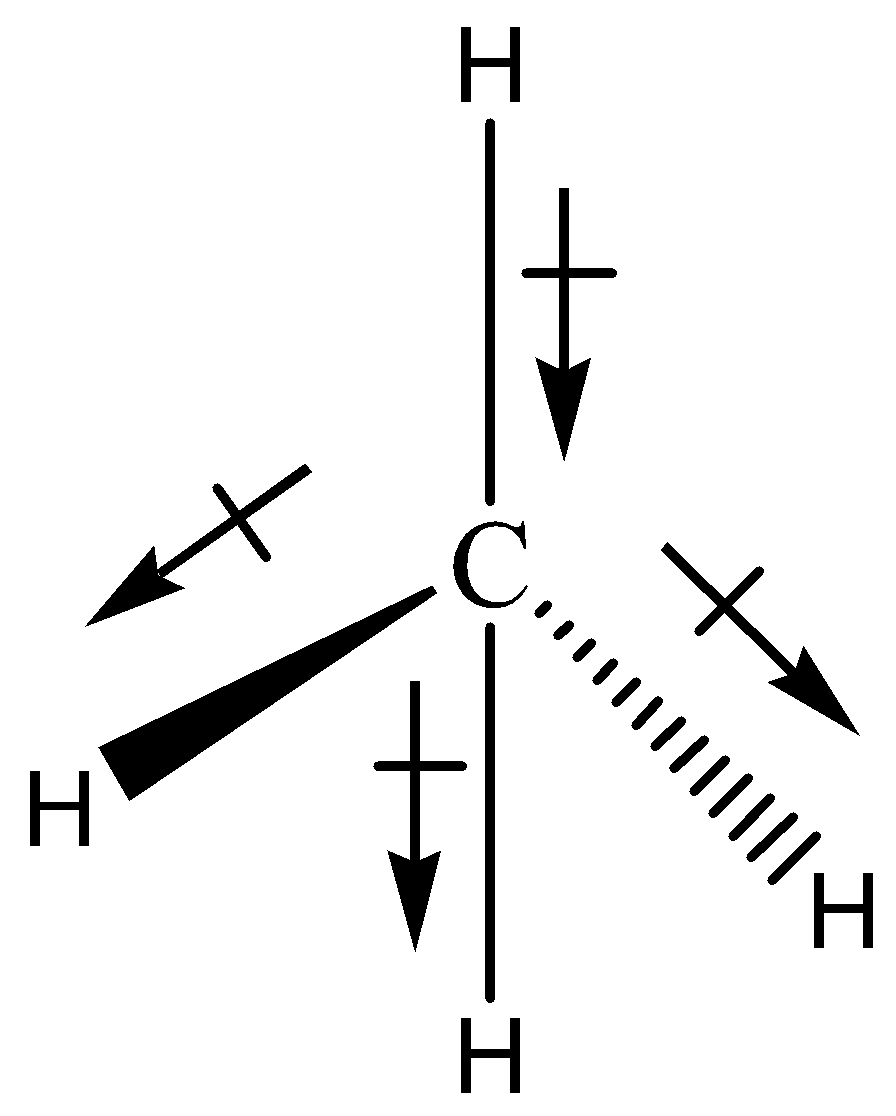
This is the reason for the polarity of $C{H_2}C{l_2}$ becoming a polar molecule. Polarity of the $C{H_2}C{l_2}$ is not dependent on the geometry. The geometry of this molecule is tetrahedral. Its polarity does not depend on the tetrahedral.
Hence, the $C{H_2}C{l_2}$ molecule may be polar or nonpolar depending on its geometry is false.
Note: We must remember that if the polarity of the molecule is dependent on the geometry means all the tetrahedral molecules become polar. But not like that nature of the polarity of the molecule is dependent only on the dipole moment. For example, methane is also geometrical. But this is highly non polar. In methane all the dipole bonds cancel each other, hence it becomes a non-polar molecule.
Complete step by step answer:
We must remember that the molecule $C{H_2}C{l_2}$ is called methylene dichloride. It is popularly called dichloromethane. Simply called DCM. It is one of the highly polar solvent in organic chemistry.
As we know that in $C{H_2}C{l_2}$ having four dipole bonds. The two dipole bonds are between $C - H$ . But these two dipole moments are present in opposite cancel each other. Other two dipole bonds are between $C - Cl$ . These two dipole bonds are given a net dipole moment of the molecule. These two $C - Cl$ dipole bonds do not cancel each other because they are present in the same direction in the molecule.
The above discussion is easily understand by its molecule diagram is given below,

This is the reason for the polarity of $C{H_2}C{l_2}$ becoming a polar molecule. Polarity of the $C{H_2}C{l_2}$ is not dependent on the geometry. The geometry of this molecule is tetrahedral. Its polarity does not depend on the tetrahedral.
Hence, the $C{H_2}C{l_2}$ molecule may be polar or nonpolar depending on its geometry is false.
Note: We must remember that if the polarity of the molecule is dependent on the geometry means all the tetrahedral molecules become polar. But not like that nature of the polarity of the molecule is dependent only on the dipole moment. For example, methane is also geometrical. But this is highly non polar. In methane all the dipole bonds cancel each other, hence it becomes a non-polar molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




