
State true or false.
${\text{S}}{{\text{F}}_{\text{6}}}$ molecule is octahedral.
(A) True
(B) False
Answer
579k+ views
Hint: Draw the structure of the molecules using the Lewis dot structure. From the structure, determine the geometry of the ${\text{S}}{{\text{F}}_{\text{6}}}$ molecule.
Complete step by step answer:
Calculate the valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ molecule as follows:
The valence electrons of ${\text{S}}$ are ${\text{6}}$ and the valence electrons of ${\text{F}}$ are ${\text{7}}$. Thus,
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $= \left( {1 \times {\text{Valence electrons of S}}} \right) + \left( {6 \times {\text{Valence electrons of F}}} \right)$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = \left( {1 \times 6} \right) + \left( {6 \times 7} \right)$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = 6 + 42$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = {\text{48}}$
Draw the Lewis structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ as follows:
The structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ is,
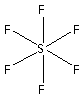
The six fluorine atoms bond with one sulphur atom forming six bonds. As six bonds are formed, twelve electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons $ = 48 - 12 = 36$
Place the remaining ${\text{36}}$ electrons around the fluorine atoms such that all the six fluorine atoms complete their octets. If still electrons remain then place them around the central sulphur atom. Thus,
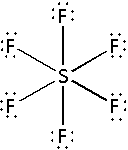
The structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ has six electron bond pairs and no electron lone pair on the central sulphur atom. Thus, the shape of ${\text{S}}{{\text{F}}_{\text{6}}}$ molecule is octahedral.
Thus, the statement ‘${\text{S}}{{\text{F}}_{\text{6}}}$ molecule is octahedral’ is true.
Thus, the correct option is (A) true.
Note: Steps to predict the geometry of a molecule are as follows:
-Determine the number of total valence electrons. (The number of valence electrons of an atom is equal to the number of the group in which the atom lies.)
-Determine the number of electrons involved in bonding.
-Determine the number of remaining electrons.
-Draw the Lewis structure.
-Determine the number of bonding groups and the number of lone pairs of electrons on the central atom.
-Predict the geometry.
Complete step by step answer:
Calculate the valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ molecule as follows:
The valence electrons of ${\text{S}}$ are ${\text{6}}$ and the valence electrons of ${\text{F}}$ are ${\text{7}}$. Thus,
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $= \left( {1 \times {\text{Valence electrons of S}}} \right) + \left( {6 \times {\text{Valence electrons of F}}} \right)$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = \left( {1 \times 6} \right) + \left( {6 \times 7} \right)$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = 6 + 42$
Valence electrons of ${\text{S}}{{\text{F}}_{\text{6}}}$ $ = {\text{48}}$
Draw the Lewis structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ as follows:
The structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ is,

The six fluorine atoms bond with one sulphur atom forming six bonds. As six bonds are formed, twelve electrons are involved in bonding. Thus, the remaining electrons are,
Remaining electrons $ = 48 - 12 = 36$
Place the remaining ${\text{36}}$ electrons around the fluorine atoms such that all the six fluorine atoms complete their octets. If still electrons remain then place them around the central sulphur atom. Thus,

The structure of ${\text{S}}{{\text{F}}_{\text{6}}}$ has six electron bond pairs and no electron lone pair on the central sulphur atom. Thus, the shape of ${\text{S}}{{\text{F}}_{\text{6}}}$ molecule is octahedral.
Thus, the statement ‘${\text{S}}{{\text{F}}_{\text{6}}}$ molecule is octahedral’ is true.
Thus, the correct option is (A) true.
Note: Steps to predict the geometry of a molecule are as follows:
-Determine the number of total valence electrons. (The number of valence electrons of an atom is equal to the number of the group in which the atom lies.)
-Determine the number of electrons involved in bonding.
-Determine the number of remaining electrons.
-Draw the Lewis structure.
-Determine the number of bonding groups and the number of lone pairs of electrons on the central atom.
-Predict the geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




