
State the law of conservation of mass. State the main points of Landolt's experiment for experimental evidence of the law.
Answer
612.6k+ views
Hint- Here, we will proceed further by understanding the conversion of mass from one state to another state then we will proceed to discuss Landolt's experiment and find some key points.
Complete answer:
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law, the mass of the products in a chemical reaction must equal the mass of the reactants. The law is useful for a number of calculations and can be used to solve for unknown masses, such as the amount of gas consumed or produced during a reaction.
Diagram:
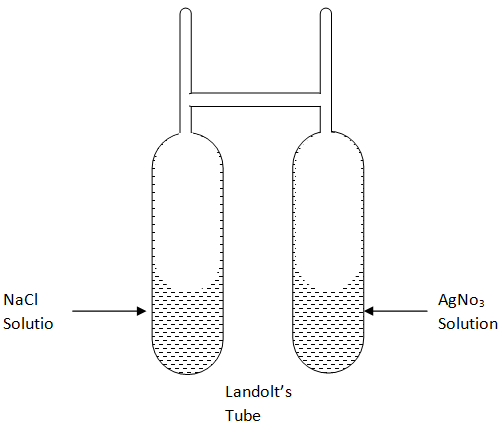
Main points of Landolt's Experiment were:-
(1) A specially designed H-shaped tube is taken with its one limb having Sodium chloride solution and the other has AgNO3 . This, in the first place, was weighed after sealing.
(2) The reaction was made to take place and then the tube was weighed.
(3) The mass of the tube in both cases was found to be the same. Thus redefining the Law of Conservation of Mass.
Note- The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such as the amount of gas consumed or produced during a reaction. Students must remember the law of conservation of mass as it has various applications in every field.
Complete answer:
The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law, the mass of the products in a chemical reaction must equal the mass of the reactants. The law is useful for a number of calculations and can be used to solve for unknown masses, such as the amount of gas consumed or produced during a reaction.
Diagram:

Main points of Landolt's Experiment were:-
(1) A specially designed H-shaped tube is taken with its one limb having Sodium chloride solution and the other has AgNO3 . This, in the first place, was weighed after sealing.
(2) The reaction was made to take place and then the tube was weighed.
(3) The mass of the tube in both cases was found to be the same. Thus redefining the Law of Conservation of Mass.
Note- The law of conservation of mass is useful for a number of calculations and can be used to solve for unknown masses, such as the amount of gas consumed or produced during a reaction. Students must remember the law of conservation of mass as it has various applications in every field.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




