
State the importance of pH in the digestion of food.
Answer
581.7k+ views
Hint: pH is basically defined as the negative logarithm of ${H^ + }$ ion concentration. The pH of the solution generally varies from 0-14. The solutions having a pH ranging from 0-7 on pH scale are termed as acidic and for the value of pH ranging 7-14 on the scale are known as basic solutions.
Complete step by step answer:
As we know that all the acids and bases do not react with the same chemical compound at the same rate. So to determine the strength of acids and bases, we use the universal indicator which shows different colors at different concentrations of hydrogen ion in solution and generally the value of pH of acids and bases are used to quantitatively determine their strength. It is basically the measure of acidity or alkalinity of a solution. The pH scale is as shown:
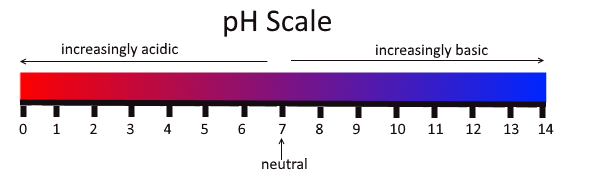
Now, our stomach produces HCl which helps in the digestion of food without harming the stomach. During indigestion, the stomach produces too much acid and this causes pain and irritation. So, to get rid of this pain, people use bases known as antacids. These antacids neutralize the excess acid in the stomach. For example, magnesium hydroxide i.e. milk of magnesia, a mild base is often used for this purpose.
Moreover, when the pH level in the mouth begins to fall below 5.5, tooth decay occurs. When the mouth is exposed to long periods of low pH, it allows cavities causing bacteria to grow further.
Note: We experience a lot of pain in case of bee-sting as the bee injects methanolic acid through its sting. So, we are generally applying baking soda or other mild bases on the surface as it helps in maintaining the pH of the surface.
Complete step by step answer:
As we know that all the acids and bases do not react with the same chemical compound at the same rate. So to determine the strength of acids and bases, we use the universal indicator which shows different colors at different concentrations of hydrogen ion in solution and generally the value of pH of acids and bases are used to quantitatively determine their strength. It is basically the measure of acidity or alkalinity of a solution. The pH scale is as shown:

Now, our stomach produces HCl which helps in the digestion of food without harming the stomach. During indigestion, the stomach produces too much acid and this causes pain and irritation. So, to get rid of this pain, people use bases known as antacids. These antacids neutralize the excess acid in the stomach. For example, magnesium hydroxide i.e. milk of magnesia, a mild base is often used for this purpose.
Moreover, when the pH level in the mouth begins to fall below 5.5, tooth decay occurs. When the mouth is exposed to long periods of low pH, it allows cavities causing bacteria to grow further.
Note: We experience a lot of pain in case of bee-sting as the bee injects methanolic acid through its sting. So, we are generally applying baking soda or other mild bases on the surface as it helps in maintaining the pH of the surface.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




