
State reasons for the following:
$ S{F_6} $ is kinetically inert substance
Answer
546.3k+ views
Hint: Sulphur hexafluoride ( $ S{F_6} $ ) is an inorganic, colourless, odourless, non-inflammable, non-toxic and hypervalent molecule. It is usually used as an electrical insulator and arc suppressant. It has a density higher than air and is usually transported as liquefied compressed gas.
Complete step by step solution:
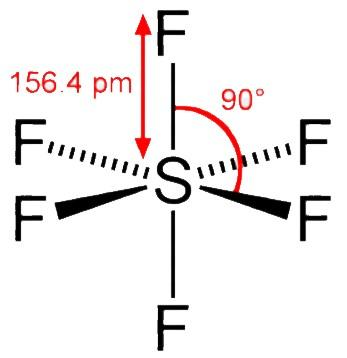
Sulphur hexafluoride ( $ S{F_6} $ ) has an octahedral geometry where the sulphur atom is being surrounded by six fluorine atoms. Sulphur hexafluoride ( $ S{F_6} $ ) is usually prepared by exposing sulphur ( $ {S_8} $ ) to fluorine ( $ {F_2} $ ). It is an inorganic non - polar gas. $ S{F_6} $ is $ s{p^3}{d^2} $ hybridised and during its formation the central sulphur atom is in its ground state having $ 3{s^2}3{p^4} $ configuration. It consists of six $ s{p^3}{d^2} $ hybridised orbitals that are projected towards six corners of a regular octahedron.
In $ S{F_6} $ the central sulphur atom is sterically surrounded by the protection of six fluorine ( $ F $ ) atoms where the $ F - S - F $ bonds are slated to be at an angle of $ 90^\circ $ . The fluorine atom surrounding the central sulphur atom protects the sulphur atom without allowing the water molecules to get in. Also, fluorine doesn’t have vacant $ d - $ orbitals to accept the electron which is being donated by $ {H_2}O $ .
Thus $ S{F_6} $ stays to be kinetically inert.
Note:
$ S{F_6} $ is a type of greenhouse gas which is colourless, odourless, non-toxic and non – flammable. It is inert in nature in the troposphere and stratosphere. $ S{F_6} $ is commonly used as a high voltage dielectric in high voltage supplies.
Complete step by step solution:
Sulphur hexafluoride ( $ S{F_6} $ ) has an octahedral geometry where the sulphur atom is being surrounded by six fluorine atoms. Sulphur hexafluoride ( $ S{F_6} $ ) is usually prepared by exposing sulphur ( $ {S_8} $ ) to fluorine ( $ {F_2} $ ). It is an inorganic non - polar gas. $ S{F_6} $ is $ s{p^3}{d^2} $ hybridised and during its formation the central sulphur atom is in its ground state having $ 3{s^2}3{p^4} $ configuration. It consists of six $ s{p^3}{d^2} $ hybridised orbitals that are projected towards six corners of a regular octahedron.
In $ S{F_6} $ the central sulphur atom is sterically surrounded by the protection of six fluorine ( $ F $ ) atoms where the $ F - S - F $ bonds are slated to be at an angle of $ 90^\circ $ . The fluorine atom surrounding the central sulphur atom protects the sulphur atom without allowing the water molecules to get in. Also, fluorine doesn’t have vacant $ d - $ orbitals to accept the electron which is being donated by $ {H_2}O $ .
Thus $ S{F_6} $ stays to be kinetically inert.

Note:
$ S{F_6} $ is a type of greenhouse gas which is colourless, odourless, non-toxic and non – flammable. It is inert in nature in the troposphere and stratosphere. $ S{F_6} $ is commonly used as a high voltage dielectric in high voltage supplies.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




