
State one uses each of DDT and iodoform. Which compound in the following couple will react faster in $S_N2$ displacement and why?
i)1-Bromopentane or 2-bromopentane
ii)1-Bromo-2methylbutane or 2-Bromo-2-methylbutane
Answer
571.2k+ views
Hint: In the first part of the question we are asked about the uses of DDT and iodoform. In the second part we are asked about the one which will react faster in $S_N2$ displacement reaction. There are many factors which determine this.
Complete step by step solution:
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochlorine. The use of DDT is that it is used as an insecticide.
Iodoform is an organic iodine compound with the formula CHI3 and a tetrahedral molecular geometry. It is a relatively water-insoluble yellow solid that is chemically reactive in free-radical reactions. The use of iodoform is that it is used as a reagent to distinguish between organic compounds commonly known as iodoform test. It is also used as an antiseptic.
In the second part of the question we are asked about the rate of $S_N2$ reaction. We will consider the factors which determine the rate of $S_N2$ reaction. One of the factors by which we will solve this question is steric hindrance.
On the basis of this factor $S_N2$ reaction will be fastest in primary compound followed by secondary compound then tertiary compound.
The structure of 1-Bromopentane is
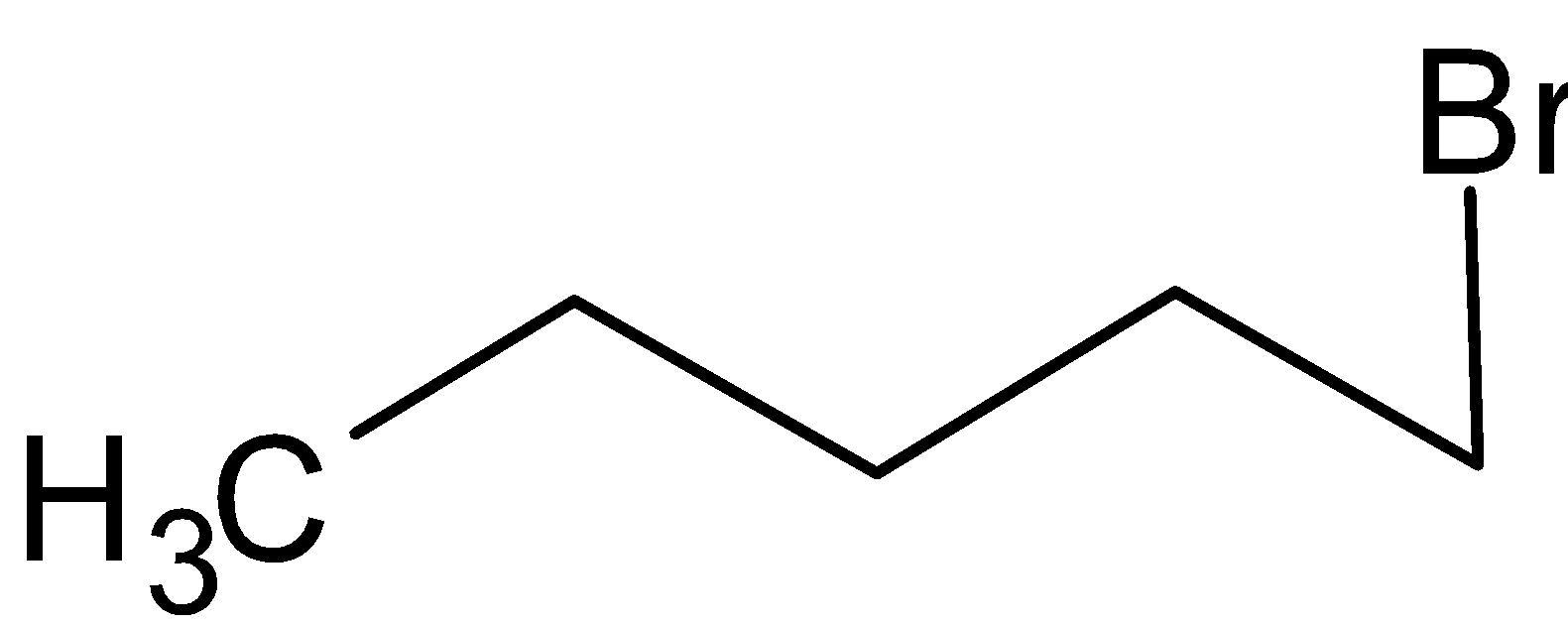
The structure of 2-Bromopentane is
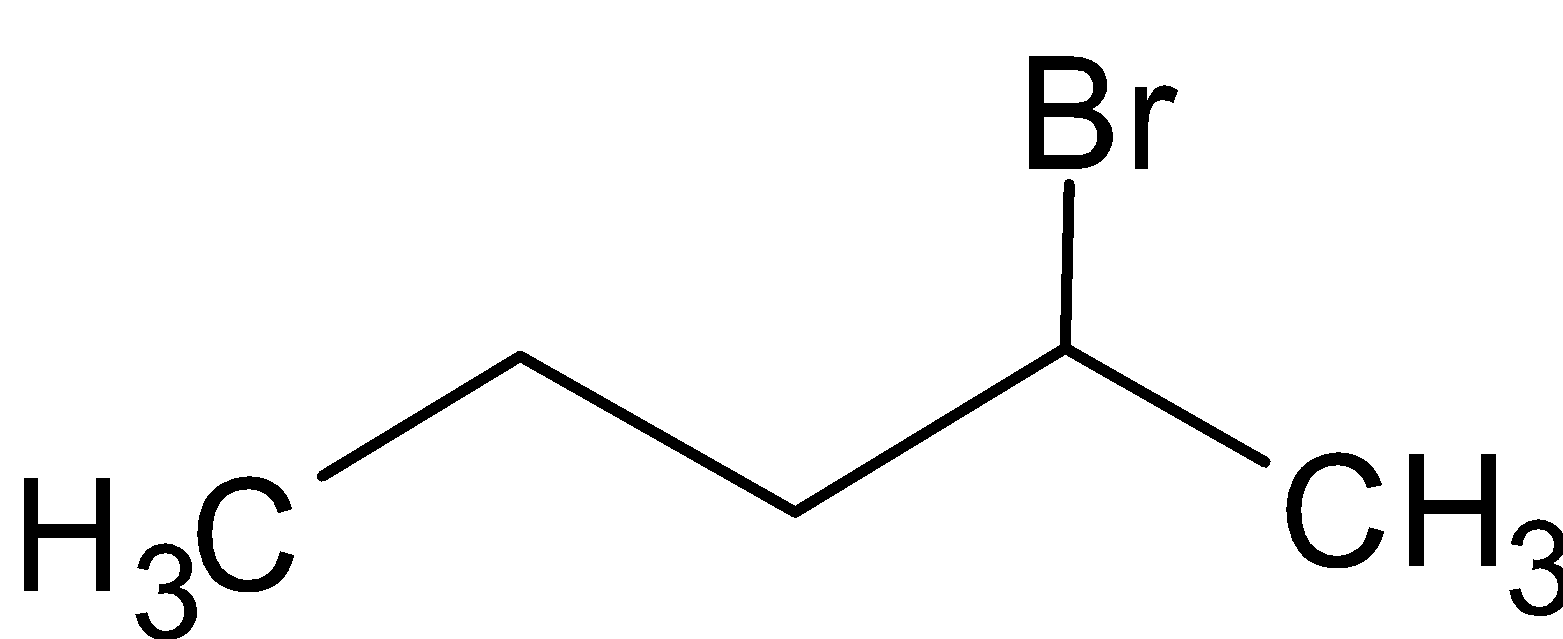
The rate of reaction will be faster in 1-bromopentane.
In the second one we have 1-Bromo-2-methylbutane or 2-Bromo-2-methylbutane
The structure of 1-Bromo-2-methylbutane is
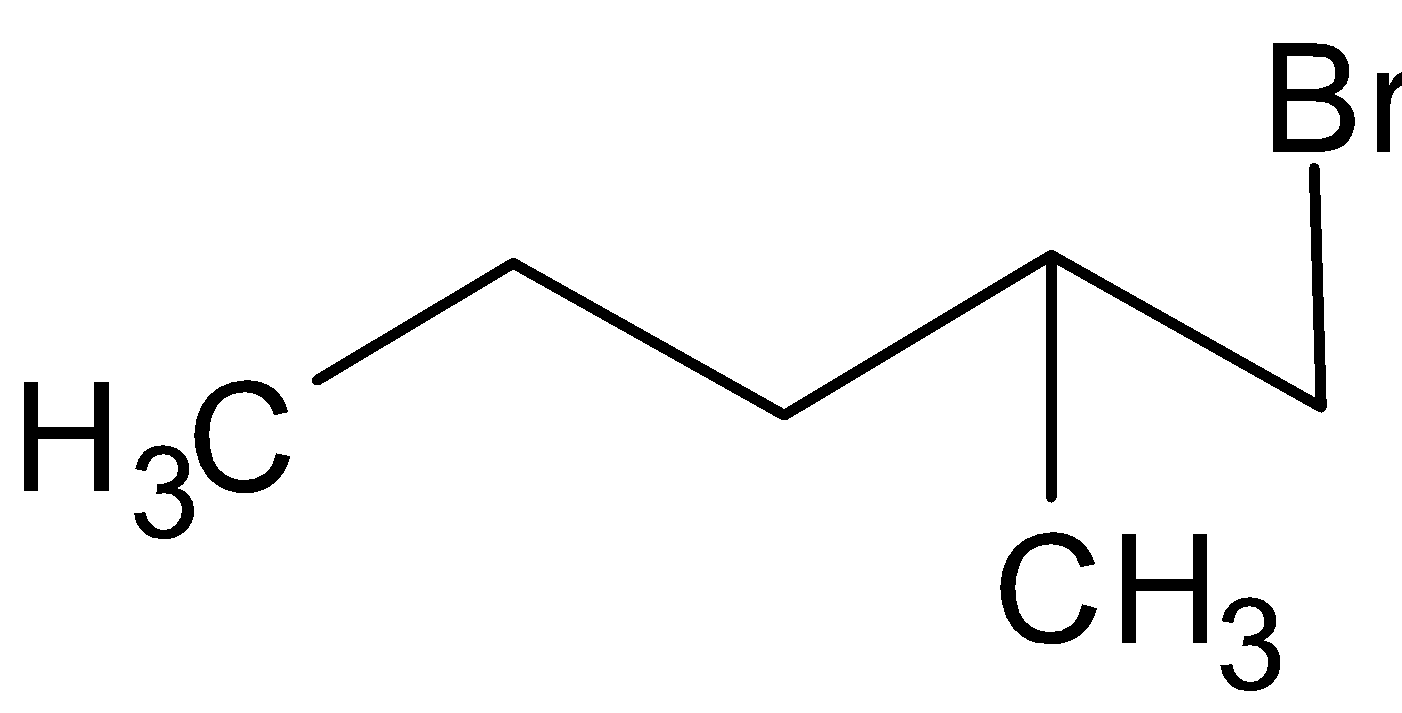
The structure of 2-Bromo-2-methylbutane is
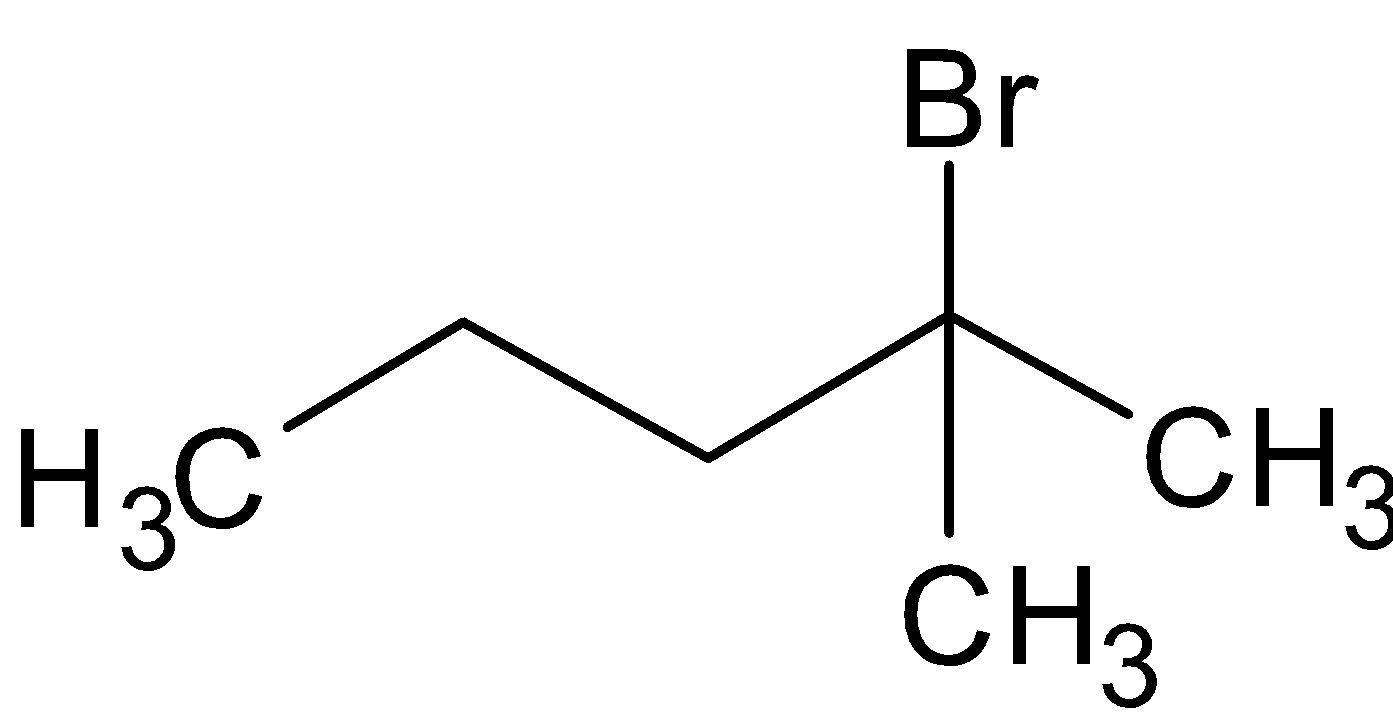
The rate of reaction will be faster in 1-Bromo-2-methylbutane as it has primary halide.
Note: In the above question we used the factors which determine $S_N2$ reaction and got the answer. The factors should be known to solve this type of question. Also an easier step will be to remove the halide atom and check the carbacation present.
Complete step by step solution:
Dichlorodiphenyltrichloroethane, commonly known as DDT, is a colorless, tasteless, and almost odorless crystalline chemical compound, an organochlorine. The use of DDT is that it is used as an insecticide.
Iodoform is an organic iodine compound with the formula CHI3 and a tetrahedral molecular geometry. It is a relatively water-insoluble yellow solid that is chemically reactive in free-radical reactions. The use of iodoform is that it is used as a reagent to distinguish between organic compounds commonly known as iodoform test. It is also used as an antiseptic.
In the second part of the question we are asked about the rate of $S_N2$ reaction. We will consider the factors which determine the rate of $S_N2$ reaction. One of the factors by which we will solve this question is steric hindrance.
On the basis of this factor $S_N2$ reaction will be fastest in primary compound followed by secondary compound then tertiary compound.
The structure of 1-Bromopentane is

The structure of 2-Bromopentane is

The rate of reaction will be faster in 1-bromopentane.
In the second one we have 1-Bromo-2-methylbutane or 2-Bromo-2-methylbutane
The structure of 1-Bromo-2-methylbutane is

The structure of 2-Bromo-2-methylbutane is

The rate of reaction will be faster in 1-Bromo-2-methylbutane as it has primary halide.
Note: In the above question we used the factors which determine $S_N2$ reaction and got the answer. The factors should be known to solve this type of question. Also an easier step will be to remove the halide atom and check the carbacation present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




