
State Faraday’s First law of electrolysis. Write its mathematical form using usual notations.
Answer
581.1k+ views
Hint: A detailed research on the electrolysis of solutions and electrolyte melts was performed by Michael Faraday. He demonstrated the mathematical implications of electrolysis using some laws. The laws formulated by Faraday are termed as the first law of electrolysis and the second law of electrolysis.
Complete step by step answer:
Before talking about the laws of electrolysis let’s first learn about the process of Electrolysis.
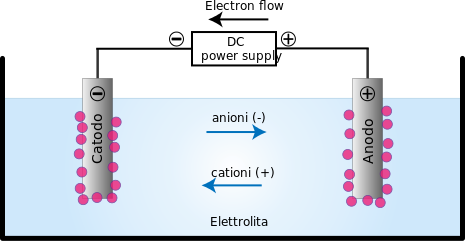
Electrolysis: It is the process of decomposition of an electrolyte when an electric current is passed through its aqueous solution or fused state.
This topic is something that is interlinked with the area of electrochemistry. So in electrochemistry, electrochemical cells are functioning. Electrochemical cells are the ones that produce electricity as a result of chemical reaction, which technically means, chemical energy is converted into electrical energy. In this cell, positive and negative electrodes are dipped in an electrolytic solution which contains positive and negative ions.
Now, let’s get into the point; a law needs to be stated as such
Faraday’s first law of electrolysis
This law states that “The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte”.
If $ m $ $ grams $ the substance is deposited on passing $ Q $ $ coulombs $ of electricity for a time $ t $, then,
$ m \propto Q $ or $ m \propto I \propto t $ as $ (Q = I.t) $
$ \Rightarrow m = Z \times I \times t $
Where $ Z $ is the electrochemical equivalent
Mathematically,
\[m\; = \dfrac{{I(A) \times t(s)}}{{96500(C/mol{e^ - })}} \times \;\]\[mole{\text{ }}ratio \times \;molar{\text{ }}mass{\text{ }}of{\text{ }}the{\text{ }}substance\]
Additional Information:
Electrolysis is very commonly used in metallurgical methods, such as electro-winning which is the extraction procedure, or electro-plating or electro-refining of mineral or metal solution. The electrolysis of water produces hydrogen and oxygen.
Note:
Whenever an electrolyte is dissolved in water the molecules break into positive and negative ions. The positive ions (or metal ions) travel to the electrodes attached to the battery's negative terminal, where electrons are taken by them, becoming a pure metal atom and are then placed on the electrode. Negative ions travel to the electrode linked to the battery's positive terminal, where they give up their extra electrons.
Complete step by step answer:
Before talking about the laws of electrolysis let’s first learn about the process of Electrolysis.
Electrolysis: It is the process of decomposition of an electrolyte when an electric current is passed through its aqueous solution or fused state.
This topic is something that is interlinked with the area of electrochemistry. So in electrochemistry, electrochemical cells are functioning. Electrochemical cells are the ones that produce electricity as a result of chemical reaction, which technically means, chemical energy is converted into electrical energy. In this cell, positive and negative electrodes are dipped in an electrolytic solution which contains positive and negative ions.

Now, let’s get into the point; a law needs to be stated as such
Faraday’s first law of electrolysis
This law states that “The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte”.
If $ m $ $ grams $ the substance is deposited on passing $ Q $ $ coulombs $ of electricity for a time $ t $, then,
$ m \propto Q $ or $ m \propto I \propto t $ as $ (Q = I.t) $
$ \Rightarrow m = Z \times I \times t $
Where $ Z $ is the electrochemical equivalent
Mathematically,
\[m\; = \dfrac{{I(A) \times t(s)}}{{96500(C/mol{e^ - })}} \times \;\]\[mole{\text{ }}ratio \times \;molar{\text{ }}mass{\text{ }}of{\text{ }}the{\text{ }}substance\]
Additional Information:
Electrolysis is very commonly used in metallurgical methods, such as electro-winning which is the extraction procedure, or electro-plating or electro-refining of mineral or metal solution. The electrolysis of water produces hydrogen and oxygen.
Note:
Whenever an electrolyte is dissolved in water the molecules break into positive and negative ions. The positive ions (or metal ions) travel to the electrodes attached to the battery's negative terminal, where electrons are taken by them, becoming a pure metal atom and are then placed on the electrode. Negative ions travel to the electrode linked to the battery's positive terminal, where they give up their extra electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




