
State any two postulates of particulate nature of matter.
Answer
585.6k+ views
Hint: Matter is anything that occupies space and has mass. All matter is made up of substances called elements, which have specific physical and chemical properties and can’t be broken down into other substances through ordinary chemical reactions.
Complete answer:
Characteristics of matter are:
> The atoms and molecules can be only discussed at microscopic level; these are very tiny discrete particles.
> Particles of matter are tiny and are measured only in micrometer, nanometer or picometer because they are very tiny.
> Particles always attract each other - The molecules in solid have no intermolecular space as the molecules in solid are tightly bound by strong force of attraction this force is comparatively weaker in liquid and gas.
> All the particles are always in a constant motion. Heating the particles leads to the change in the speed of the particles as they start moving fast.
Additional Information: At the macroscopic or bulk level, matter can be classified as mixtures or pure substances.
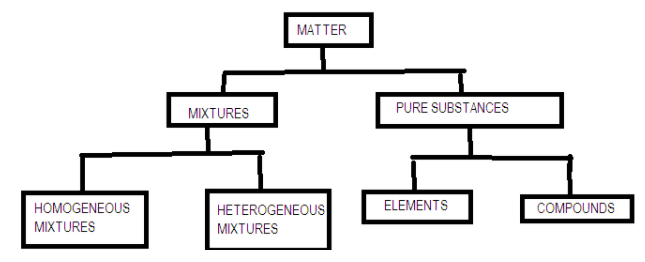
Note: Matter exists in three states namely; solid, liquid and gas. The difference arises due to the arrangement of particles in the space. Solid particles are closely packed, in liquids particles have some spaces between them but in gases the particles are too far away from each other.
Complete answer:
Characteristics of matter are:
> The atoms and molecules can be only discussed at microscopic level; these are very tiny discrete particles.
> Particles of matter are tiny and are measured only in micrometer, nanometer or picometer because they are very tiny.
> Particles always attract each other - The molecules in solid have no intermolecular space as the molecules in solid are tightly bound by strong force of attraction this force is comparatively weaker in liquid and gas.
> All the particles are always in a constant motion. Heating the particles leads to the change in the speed of the particles as they start moving fast.
Additional Information: At the macroscopic or bulk level, matter can be classified as mixtures or pure substances.

Note: Matter exists in three states namely; solid, liquid and gas. The difference arises due to the arrangement of particles in the space. Solid particles are closely packed, in liquids particles have some spaces between them but in gases the particles are too far away from each other.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




