
Starting from the Lewis structure, determine the Hybridization types of the central atom of $ TeC{l_4} $ and $ IC{l_4}^ - $ ?
Answer
497.1k+ views
Hint: Hybridization is a process of mixing orbitals of different energy to generate new hybrid orbitals of same energy as well as shape. Tellurium and iodine are members of the fifth period which have vacant $ d $ orbital.
Complete Step By Step Answer:
As we know the atomic number of tellurium is $ 52 $ so its electronic configuration will become $ \left[ {Kr} \right]4{d^{10}}5{s^2}5{p^4} $ . From the electronic configuration we can see that Tellurium has $ 6 $ electrons in its valence shell. Similarly, electronic configuration of chlorine with atomic number $ 17 $ is $ \left[ {Ne} \right]3{s^2}3{p^5} $ . From the electronic configuration we can see that the chlorine atom has $ 7 $ electrons in its valence shell.
In the molecule of $ TeC{l_4} $ , four chlorine atoms share one electron with Tellurium. Hence, four electrons of Tellurium are shared with chlorine atoms. So, the atom of tellurium has two lone pair electrons left. So finally, we say that when $ TeC{l_4} $ is formed four bonding electrons and two lone pair electrons undergo hybridization. Hence, hybridization of $ TeC{l_4} $ is $ \left( {s{p^3}d} \right) $ .
As we know the atomic number of iodine is $ 53 $ so its electronic configuration will become $ \left[ {Kr} \right]4{d^{10}}5{s^2}5{p^5} $ . From the electronic configuration we can see that iodine has $ 7 $ electrons in its valence shell.
In the molecule of $ IC{l_4}^ - $ , four chlorine atoms share one electron with the iodine atom. Hence, four electrons of iodine are shared with four chlorine atoms. So, there are $ 3 $ electrons and $ 1 $ negative charge is still remaining to the iodine atom which together form $ 4 $ electrons or $ 2 $ pair electrons. The atom of iodine has two lone pair electrons left. So finally, we say that when $ IC{l_4}^ - $ is formed by four bonding electrons and two lone pair electrons present above and below the plane will undergo hybridization. Hence, hybridization of $ IC{l_4}^ - $ is $ \left( {s{p^3}{d^2}} \right) $ .
$ \Rightarrow $ Lewis structure of $ TeC{l_4} $ and $ IC{l_4}^ - $ is:
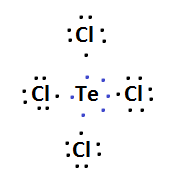
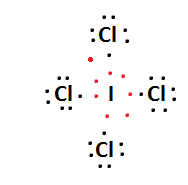
$ \Rightarrow $ Hybridization of $ TeC{l_4} $ is $ \left( {s{p^3}d} \right) $ and $ IC{l_4}^ - $ is $ \left( {s{p^3}{d^2}} \right) $
Note:
$ \left( {s{p^3}d} \right) $ hybridization is also found in the case of $ PC{l_5},P{F_5} $ and having trigonal bipyramidal geometry. $ \left( {s{p^3}{d^2}} \right) $ hybridization is also found in case of $ S{F_6},Cr{F_6}^{ - 3} $ and having octahedral geometry.
Complete Step By Step Answer:
As we know the atomic number of tellurium is $ 52 $ so its electronic configuration will become $ \left[ {Kr} \right]4{d^{10}}5{s^2}5{p^4} $ . From the electronic configuration we can see that Tellurium has $ 6 $ electrons in its valence shell. Similarly, electronic configuration of chlorine with atomic number $ 17 $ is $ \left[ {Ne} \right]3{s^2}3{p^5} $ . From the electronic configuration we can see that the chlorine atom has $ 7 $ electrons in its valence shell.
In the molecule of $ TeC{l_4} $ , four chlorine atoms share one electron with Tellurium. Hence, four electrons of Tellurium are shared with chlorine atoms. So, the atom of tellurium has two lone pair electrons left. So finally, we say that when $ TeC{l_4} $ is formed four bonding electrons and two lone pair electrons undergo hybridization. Hence, hybridization of $ TeC{l_4} $ is $ \left( {s{p^3}d} \right) $ .
As we know the atomic number of iodine is $ 53 $ so its electronic configuration will become $ \left[ {Kr} \right]4{d^{10}}5{s^2}5{p^5} $ . From the electronic configuration we can see that iodine has $ 7 $ electrons in its valence shell.
In the molecule of $ IC{l_4}^ - $ , four chlorine atoms share one electron with the iodine atom. Hence, four electrons of iodine are shared with four chlorine atoms. So, there are $ 3 $ electrons and $ 1 $ negative charge is still remaining to the iodine atom which together form $ 4 $ electrons or $ 2 $ pair electrons. The atom of iodine has two lone pair electrons left. So finally, we say that when $ IC{l_4}^ - $ is formed by four bonding electrons and two lone pair electrons present above and below the plane will undergo hybridization. Hence, hybridization of $ IC{l_4}^ - $ is $ \left( {s{p^3}{d^2}} \right) $ .
$ \Rightarrow $ Lewis structure of $ TeC{l_4} $ and $ IC{l_4}^ - $ is:
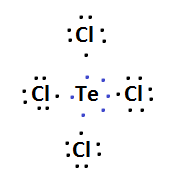

$ \Rightarrow $ Hybridization of $ TeC{l_4} $ is $ \left( {s{p^3}d} \right) $ and $ IC{l_4}^ - $ is $ \left( {s{p^3}{d^2}} \right) $
Note:
$ \left( {s{p^3}d} \right) $ hybridization is also found in the case of $ PC{l_5},P{F_5} $ and having trigonal bipyramidal geometry. $ \left( {s{p^3}{d^2}} \right) $ hybridization is also found in case of $ S{F_6},Cr{F_6}^{ - 3} $ and having octahedral geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




