
Spin multiplicity of triplet nitrene is:
(a) 1
(b) 2
(c) 3
(d) 4
Answer
604.2k+ views
Hint: To answer this question you should know that the formula which is generally used for the prediction of spin multiplicity value is (2S+1). Now you may have some idea about this term and you can predict the value for triplet nitrene.
Complete step by step answer:
First, we should know about the singlet and triplet excited states.
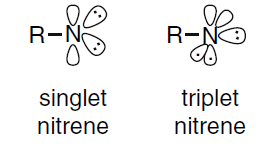
Singlet and triplet is derived using the equation for multiplicity, 2S+1
2S+1, where S is the total spin angular momentum (sum of all the electron spins). Individual spins are denoted as spin up (s=+1/2) or spin down (s=−1/2). If we were to calculate the S for the excited singlet state, the equation would be 2[(+1/2)+(−1/2)]+1=2(0)+1 = 1, therefore making the center orbital in the figure a singlet state.
If the spin multiplicity for the excited triplet state was calculated, we obtain 2[(+1/2)+(+1/2)]+1=2(1)+1 = 3, which gives a triplet state as expected.
Therefore, when nitrene is in the triplet state, its spin multiplicity = 3. Which makes option C the correct option.
Note: Let’s talk about some differences between singlet and triplet excited states.
A singlet or a triplet can form when one electron is excited to a higher energy level. In an excited singlet state, the electron is promoted in the same spin orientation as it was in the ground state (paired). In a triplet excited state, the electron that is promoted has the same spin orientation (parallel) to the other unpaired electron.
Complete step by step answer:
First, we should know about the singlet and triplet excited states.
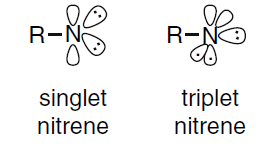
Singlet and triplet is derived using the equation for multiplicity, 2S+1
2S+1, where S is the total spin angular momentum (sum of all the electron spins). Individual spins are denoted as spin up (s=+1/2) or spin down (s=−1/2). If we were to calculate the S for the excited singlet state, the equation would be 2[(+1/2)+(−1/2)]+1=2(0)+1 = 1, therefore making the center orbital in the figure a singlet state.
If the spin multiplicity for the excited triplet state was calculated, we obtain 2[(+1/2)+(+1/2)]+1=2(1)+1 = 3, which gives a triplet state as expected.
Therefore, when nitrene is in the triplet state, its spin multiplicity = 3. Which makes option C the correct option.
Note: Let’s talk about some differences between singlet and triplet excited states.
A singlet or a triplet can form when one electron is excited to a higher energy level. In an excited singlet state, the electron is promoted in the same spin orientation as it was in the ground state (paired). In a triplet excited state, the electron that is promoted has the same spin orientation (parallel) to the other unpaired electron.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




