
What is spectrochemical series and what is its importance?
Answer
551.4k+ views
Hint: In coordination chemistry a ligand can be defined as an ion or molecule that binds with a central metal atom to form a coordination complex. The bonding with the metal generally involves the donation of one or more electron pairs.
Complete answer:
Spectrochemical series was founded in 1938 which is a list of ligands according to their strength or we can say a list of metal ions based on their oxidation number, group and identity. We know that in crystal field theory ligands modify the difference in energy between the d orbitals known as crystal field splitting.
Ligands are generally known as electron donors and metals are electron acceptors. Ligands are of two types: weak field ligands and strong field ligands. Whereas strong field ligands are those ligands which produce a large crystal field splitting which further leads for low spin value and on the other hand weak field ligands are those ligands which have low crystal field splitting and leads for high value.
A series which arrange the ligands from large splitting value to small splitting value is known as spectrochemical series which can be shown as:
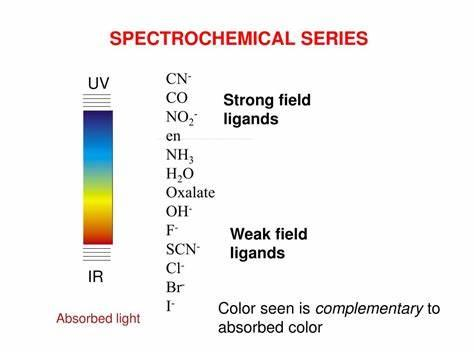
It is important to know easily about weak field and strong field ligands.
Note:
Nature of metal to ligand bonding generally ranges from covalent to ionic and also ligand bond order can also range from 1-3. Ligands can be classified into different categories on the basis of charge, size, identity of coordinating atoms, denticity or hapticipity.
Complete answer:
Spectrochemical series was founded in 1938 which is a list of ligands according to their strength or we can say a list of metal ions based on their oxidation number, group and identity. We know that in crystal field theory ligands modify the difference in energy between the d orbitals known as crystal field splitting.
Ligands are generally known as electron donors and metals are electron acceptors. Ligands are of two types: weak field ligands and strong field ligands. Whereas strong field ligands are those ligands which produce a large crystal field splitting which further leads for low spin value and on the other hand weak field ligands are those ligands which have low crystal field splitting and leads for high value.
A series which arrange the ligands from large splitting value to small splitting value is known as spectrochemical series which can be shown as:

It is important to know easily about weak field and strong field ligands.
Note:
Nature of metal to ligand bonding generally ranges from covalent to ionic and also ligand bond order can also range from 1-3. Ligands can be classified into different categories on the basis of charge, size, identity of coordinating atoms, denticity or hapticipity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




