
How many $s{p^3}$ hybridised carbon atoms are in Acetophenone?
Answer
582.6k+ views
Hint:
Acetophenone is an organic compound. Acetophenone is the simplest aromatic ketone with the chemical formula \[{C_6}{H_5}C\left( O \right)C{H_3}\]. $s{p^3}$ hybridisation is formed by the intermixing of one $s$ orbital and three $p$ orbitals.
Complete step by step solution
The concept of hybridisation is given by Pauling. According to Pauling the atomic orbitals having slightly different energy and shape are intermixed to form hybrid orbital. These hybrid orbitals are involved in bond formation and these hybrid orbitals are equivalent in energy.
$sp$ hybridisation-In this hybridisation one $s$ and one $p$ orbitals intermixes. Due to this intermixing two equivalent $sp$ hybrid orbitals are formed. Similarly, in $s{p^2}$ hybridisation one \[s\] and two \[p\] orbitals intermix. Due to this intermixing three equivalent $s{p^2}$ hybrid orbitals are formed .Similarly in case of $s{p^3}$ hybridisation one \[s\] and one $p$ orbitals intermixes. Due to this intermixing four equivalent $s{p^3}$ hybrid orbitals are formed.
Acetophenone has the chemical formula ${C_6}{H_5}C\left( O \right)C{H_3}$.
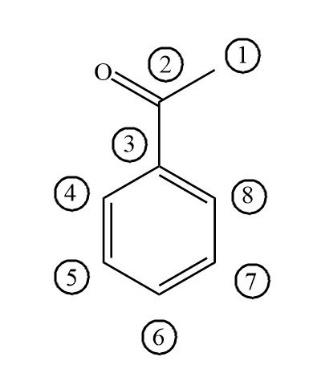
Above is the figure of Acetophenone. It has eight carbon atoms, in which six carbon atoms involved in the formation of benzene. Then one carbon is involved in the ketone functional group and the other carbon atom is involved in methyl.
First take a look at all benzene involved carbons i.e. the carbon numbers $3,4,5,6,7,8$ .All these six carbons have $3$ sigma bonds and $1$ pi bonds connected to carbon atoms. So, the hybridisation of all six carbons which is involved in benzene is $s{p^2}$ hybridised. Now take a look at carbonyl carbon i.e. carbon number $2$ which has \[3\] sigma bonds and \[1\] pi bonds connected to oxygen.
Now take a look at carbon number $1$,which is connected to one carbonyl carbon and three hydrogen atoms. So, the hybridisation of carbon $1$ becomes $s{p^3}$ hybridised.
Hence only one carbon i.e. carbon $1$ becomes $s{p^3}$ hybridised.
Note:
Thus in case of $sp$ hybridisation one $s$ and one \[p\] orbital involved so $sp$ hybridisation has $50\% $s and $50\% $\[p\] character. Similarly in $s{p^2}$hybridisation one $s$ and two \[p\] orbitals are involved so $s{p^2}$hybridisation has $33.33\% $$s$ and $66.66\% $\[p\] character. While $s{p^3}$ in hybridisation one $s$ and three \[p\] orbitals are involved so $s{p^3}$ hybridisation has $25\% $$s$ and $75\% $ $75\% $\[p\] character.
Acetophenone is an organic compound. Acetophenone is the simplest aromatic ketone with the chemical formula \[{C_6}{H_5}C\left( O \right)C{H_3}\]. $s{p^3}$ hybridisation is formed by the intermixing of one $s$ orbital and three $p$ orbitals.
Complete step by step solution
The concept of hybridisation is given by Pauling. According to Pauling the atomic orbitals having slightly different energy and shape are intermixed to form hybrid orbital. These hybrid orbitals are involved in bond formation and these hybrid orbitals are equivalent in energy.
$sp$ hybridisation-In this hybridisation one $s$ and one $p$ orbitals intermixes. Due to this intermixing two equivalent $sp$ hybrid orbitals are formed. Similarly, in $s{p^2}$ hybridisation one \[s\] and two \[p\] orbitals intermix. Due to this intermixing three equivalent $s{p^2}$ hybrid orbitals are formed .Similarly in case of $s{p^3}$ hybridisation one \[s\] and one $p$ orbitals intermixes. Due to this intermixing four equivalent $s{p^3}$ hybrid orbitals are formed.
Acetophenone has the chemical formula ${C_6}{H_5}C\left( O \right)C{H_3}$.

Above is the figure of Acetophenone. It has eight carbon atoms, in which six carbon atoms involved in the formation of benzene. Then one carbon is involved in the ketone functional group and the other carbon atom is involved in methyl.
First take a look at all benzene involved carbons i.e. the carbon numbers $3,4,5,6,7,8$ .All these six carbons have $3$ sigma bonds and $1$ pi bonds connected to carbon atoms. So, the hybridisation of all six carbons which is involved in benzene is $s{p^2}$ hybridised. Now take a look at carbonyl carbon i.e. carbon number $2$ which has \[3\] sigma bonds and \[1\] pi bonds connected to oxygen.
Now take a look at carbon number $1$,which is connected to one carbonyl carbon and three hydrogen atoms. So, the hybridisation of carbon $1$ becomes $s{p^3}$ hybridised.
Hence only one carbon i.e. carbon $1$ becomes $s{p^3}$ hybridised.
Note:
Thus in case of $sp$ hybridisation one $s$ and one \[p\] orbital involved so $sp$ hybridisation has $50\% $s and $50\% $\[p\] character. Similarly in $s{p^2}$hybridisation one $s$ and two \[p\] orbitals are involved so $s{p^2}$hybridisation has $33.33\% $$s$ and $66.66\% $\[p\] character. While $s{p^3}$ in hybridisation one $s$ and three \[p\] orbitals are involved so $s{p^3}$ hybridisation has $25\% $$s$ and $75\% $ $75\% $\[p\] character.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




