
Some equipment and materials are given.
\[ZnS{O_4}\] solution, \[CuS{O_4}\] solution, \[Zn\] rod, \[Cu\] rod, Voltmeter, \[KCl\] solution, filter paper.
(a) Draw the diagram of the electrochemical cell which can be constructed using these equipment and materials and label the pans.
(b) Write equations of chemical reactions taking place in the two electrodes of this cell.
(Hint: reactivity\[Zn > Cu\])
Answer
546.3k+ views
Hint: Electrochemical cell is a cell or a device in which electrochemical reaction takes place. The transfer of electrons during a chemical reaction is called electrochemical reaction. Here conversion of one form of energy into another form occurs like chemical potential energy to electrical potential energy, or electrical potential energy to chemical potential energy.
Complete step by step answer:
Before understanding the diagram we have to be aware of a few things used in electrochemical cells.
Electrode: An electrode can be defined as an electrical conductor which connects the ions or electrochemical species present in solution with the external electrical circuit of the cell. Here \[Zn\] and \[Cu\] are the electrodes.
Electrolyte: An electrolyte can be defined as a solution containing free ions which acts as a conductor of charges in solution. Here \[ZnS{O_4}\] and \[CuS{O_4}\] are the electrolytes.
Salt bridge: A salt bridge is a tube which contains electrolytic solution and acts as a connection between two solutions. It is used to maintain the electrical neutrality between the electrolytes present in two different parts of the solutions. Here \[KCl\] is the salt bridge.
(a)
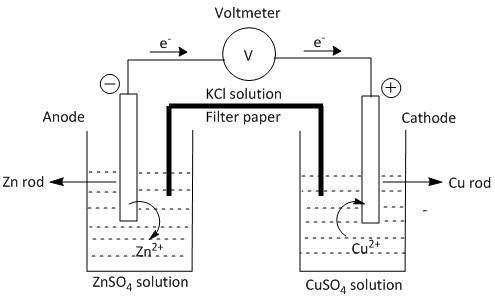
(b) Anode is defined as the electrode where oxidation takes place. Oxidation is the loss of electrons.
Cathode is the electrode where reduction takes place. Reduction is gain of electrons.
When \[Zn\] rod is dipped in \[ZnS{O_4}\] solution \[Z{n^{2 + }}\] ion goes to the solution due to the oxidation process. When \[Cu\] rod is dipped in the\[CuS{O_4}\] solution, \[C{u^{2 + }}\] ion deposits on the metal surface from the solution due to the reduction process. This is because \[C{u^{2 + }}\] ion has greater attraction towards electrons than the \[Z{n^{2 + }}\] ion. Thus the equation of the chemical reactions in the two electrodes can be written as:
At anode: \[Zn(s) \to Z{n^{2 + }}(sol) + 2{e^ - }\] (oxidation)
At cathode: $C{u^{2 + }}(sol) + 2{e^ - } \to Cu(s)$ (reduction).
Note: These cells are also known as galvanic cells. These are composed of two half cells. The electric energy or current will flow in the opposite direction to the flow of electrons. The concentration of electrons is more at anode than at cathode.
Complete step by step answer:
Before understanding the diagram we have to be aware of a few things used in electrochemical cells.
Electrode: An electrode can be defined as an electrical conductor which connects the ions or electrochemical species present in solution with the external electrical circuit of the cell. Here \[Zn\] and \[Cu\] are the electrodes.
Electrolyte: An electrolyte can be defined as a solution containing free ions which acts as a conductor of charges in solution. Here \[ZnS{O_4}\] and \[CuS{O_4}\] are the electrolytes.
Salt bridge: A salt bridge is a tube which contains electrolytic solution and acts as a connection between two solutions. It is used to maintain the electrical neutrality between the electrolytes present in two different parts of the solutions. Here \[KCl\] is the salt bridge.
(a)

(b) Anode is defined as the electrode where oxidation takes place. Oxidation is the loss of electrons.
Cathode is the electrode where reduction takes place. Reduction is gain of electrons.
When \[Zn\] rod is dipped in \[ZnS{O_4}\] solution \[Z{n^{2 + }}\] ion goes to the solution due to the oxidation process. When \[Cu\] rod is dipped in the\[CuS{O_4}\] solution, \[C{u^{2 + }}\] ion deposits on the metal surface from the solution due to the reduction process. This is because \[C{u^{2 + }}\] ion has greater attraction towards electrons than the \[Z{n^{2 + }}\] ion. Thus the equation of the chemical reactions in the two electrodes can be written as:
At anode: \[Zn(s) \to Z{n^{2 + }}(sol) + 2{e^ - }\] (oxidation)
At cathode: $C{u^{2 + }}(sol) + 2{e^ - } \to Cu(s)$ (reduction).
Note: These cells are also known as galvanic cells. These are composed of two half cells. The electric energy or current will flow in the opposite direction to the flow of electrons. The concentration of electrons is more at anode than at cathode.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




