
How soluble is potassium nitrate in water?
Answer
514.2k+ views
Hint: Potassium nitrate also known as saltpetre has a chemical formula $KN{O_3}$. It is a white solid that is soluble in water and is formed when sodium nitrate and potassium chloride solution undergo fractional distillation. It is widely used in gunpowder and in chemistry labs for preparation of nitric acid.
Complete answer: At room temperature, the structure of potassium nitrate is an orthorhombic crystal which converts to a trigonal system when the temperature rises to ${129^ \circ }C$.
Ionic compounds are more soluble in water as compared to covalent compounds and potassium nitrate i.e., $KN{O_3}$ is an ionic salt of potassium ion \[({K^ + })\] and nitrate ions \[(N{O_3}^ - )\] and when dissolved in water, the positive side of the water dipole is attracted by \[N{O_3}^ - \] ions whereas the negative side of the dipole of water is attracted by \[{K^ + }\] ions. Hence it is soluble in water.
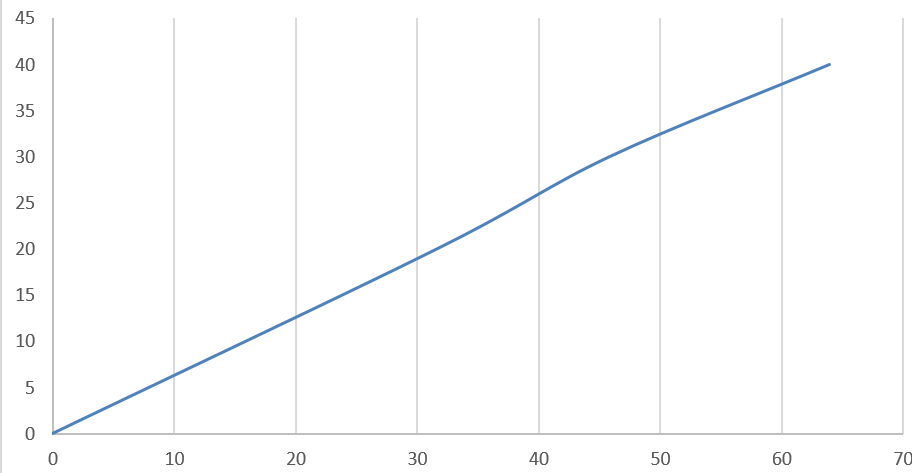
Potassium nitrate is not highly soluble in water but as the temperature increases, its solubility in water also increases. It has a tendency to absorb $0.03\% $ of water in $80\% $ relative humidity over $50$ days, hence it is not very hygroscopic i.e., it has a very less tendency to hold water molecules in its surroundings. The variation of solubility of potassium nitrate with temperature is shown as per following graph:
Note:
Potassium nitrate has many uses like it is widely used as gunpowder in the explosives like grenades, bombs, etc. It is also used in the production and manufacturing of cigarettes. It is also used in the food industry as a preservative for meat and other food products.
Complete answer: At room temperature, the structure of potassium nitrate is an orthorhombic crystal which converts to a trigonal system when the temperature rises to ${129^ \circ }C$.
Ionic compounds are more soluble in water as compared to covalent compounds and potassium nitrate i.e., $KN{O_3}$ is an ionic salt of potassium ion \[({K^ + })\] and nitrate ions \[(N{O_3}^ - )\] and when dissolved in water, the positive side of the water dipole is attracted by \[N{O_3}^ - \] ions whereas the negative side of the dipole of water is attracted by \[{K^ + }\] ions. Hence it is soluble in water.
Potassium nitrate is not highly soluble in water but as the temperature increases, its solubility in water also increases. It has a tendency to absorb $0.03\% $ of water in $80\% $ relative humidity over $50$ days, hence it is not very hygroscopic i.e., it has a very less tendency to hold water molecules in its surroundings. The variation of solubility of potassium nitrate with temperature is shown as per following graph:

Note:
Potassium nitrate has many uses like it is widely used as gunpowder in the explosives like grenades, bombs, etc. It is also used in the production and manufacturing of cigarettes. It is also used in the food industry as a preservative for meat and other food products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




