
Solid contains \[{{N}_{2}}{{O}_{5}}\]
(a) ionic
(b) covalent
(c) coordinate covalent
(d) metallic.
Answer
595.5k+ views
Hint: The bonds between a metal and nonmetal are usually ionic. Bonds between non-metal and nonmetal are covalent or coordinate. Bonds between a metal and metal are usually metallic.
Complete step-by-step answer:
Nitrogen and oxygen, both are non-metals. They do not form ionic bonds or metallic bonds together. So, the only possibilities are covalent and coordinate covalent bonds.
The structure of \[{{N}_{2}}{{O}_{5}}\] is as shown below
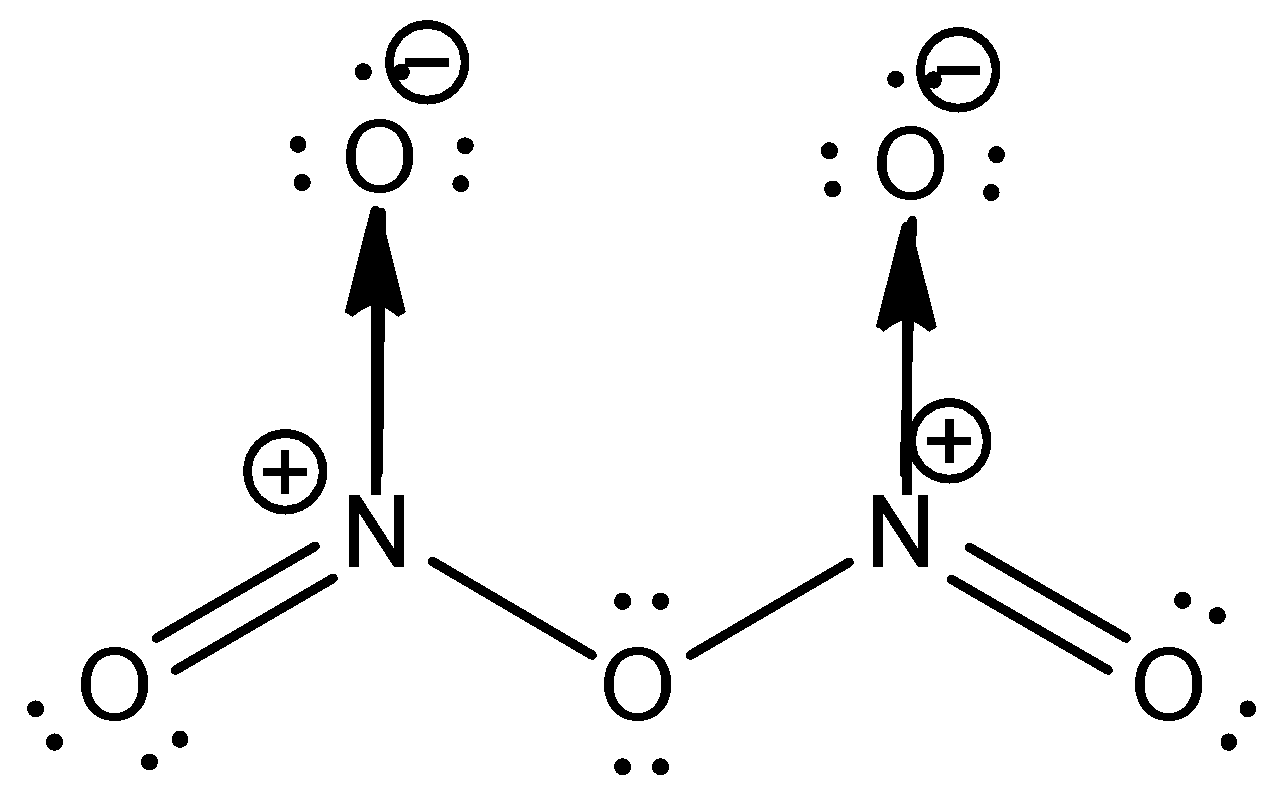
From the structure, it is clear that each nitrogen is bonded to three oxygen atoms. One oxygen atom is doubly bonded to the nitrogen; the other forms a sigma bond with nitrogen and the third oxygen atom forms a coordinate covalent bond with nitrogen. This leads to a positive charge on nitrogen. One of the oxygen connects the two nitrogen atoms.
Thus, \[{{N}_{2}}{{O}_{5}}\] have both covalent and coordinate covalent bonds. The correct answer for the question is options (b) and (c).
Additional Information: The coordinated covalent bonds are generally polar covalent bonds. These are weak bonds when compared to covalent bonds. They are long bonds with a small degree of charge transfer taking place when a bond is formed. Its preferred mode of dissociation in the gas phase is heterolytic and not homolytic. Complexes are formed by coordination bonds between metals and ligands.
Note: Coordinate covalent bonds occur when the electron pair of one of the atoms is shared with the other. Covalent bond is between in which each electron of the atoms are shared.
Complete step-by-step answer:
Nitrogen and oxygen, both are non-metals. They do not form ionic bonds or metallic bonds together. So, the only possibilities are covalent and coordinate covalent bonds.
The structure of \[{{N}_{2}}{{O}_{5}}\] is as shown below

From the structure, it is clear that each nitrogen is bonded to three oxygen atoms. One oxygen atom is doubly bonded to the nitrogen; the other forms a sigma bond with nitrogen and the third oxygen atom forms a coordinate covalent bond with nitrogen. This leads to a positive charge on nitrogen. One of the oxygen connects the two nitrogen atoms.
Thus, \[{{N}_{2}}{{O}_{5}}\] have both covalent and coordinate covalent bonds. The correct answer for the question is options (b) and (c).
Additional Information: The coordinated covalent bonds are generally polar covalent bonds. These are weak bonds when compared to covalent bonds. They are long bonds with a small degree of charge transfer taking place when a bond is formed. Its preferred mode of dissociation in the gas phase is heterolytic and not homolytic. Complexes are formed by coordination bonds between metals and ligands.
Note: Coordinate covalent bonds occur when the electron pair of one of the atoms is shared with the other. Covalent bond is between in which each electron of the atoms are shared.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




