
Sodium format on heating yields
A.Oxalic acid and \[{H_2}\]
B.Sodium oxalate and \[{H_2}\]
C. \[C{O_2}\] and NaOH
D.Sodium oxalate
Answer
574.2k+ views
Hint: Sodium format (HCOONa) is an organic sodium salt which is the monosodium salt of formic acid. It plays the role of a buffer and as well as an astringent. Sodium format contains a format. When we heat Sodium format to a temperature between 220-360 degree Celsius, decomposition reaction takes place.
Complete step by step answer:
Sodium format or HCOONa, is the sodium salt of formic acid HCOOH. It usually appears as a white deliquescent powder. On heating of sodium format, decomposition reaction occurs. As a result of this reaction sodium oxalate is formed and hydrogen is released.
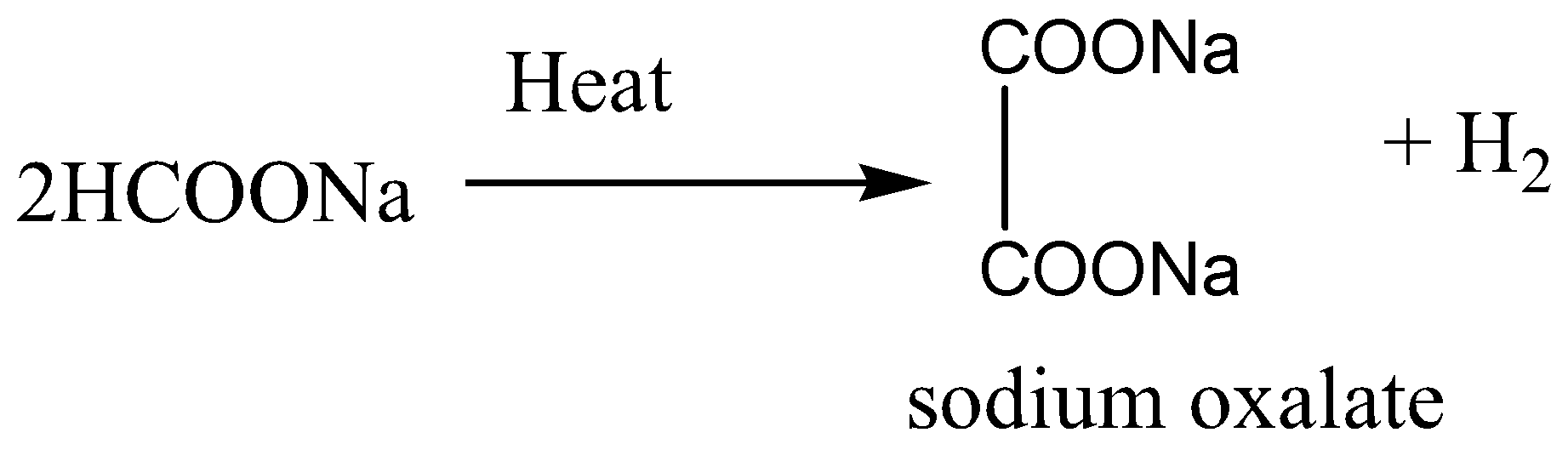
When sodium formate is heated at \[220 - {360^0}C\], a melt is formed, for getting Sodium oxalate, which is then spread thinly as a liquid over hot surfaces heated to a temperature between \[360 - {440^0}C\], where the decomposition to sodium oxalate takes place in 2 to 3 seconds with a quantitative yield conversion of format to oxalate.
If sodium formate is heated rapidly at 400 to \[{420^0}C\], the reaction time of thermal decomposition is much reduced and in case this optimum temperature is maintained, there is maximum production of sodium oxalate.
Therefore, the correct answer is option (B).
Note: Sodium formate is heated rapidly at 400 to \[{420^0}C\] that is overheating of sodium format in order to maximize the product, sodium oxalate (further decomposition of sodium oxalate), as possible to reduce the reaction time with optimum temperature of thermal decomposition. Overheating may cause decomposition of sodium oxalate.
Sodium oxalate is an organic sodium salt which consists of sodium ions and oxalate ions in a ratio of \[2:1\]. It plays the role of a poison as well as a reducing agent. It is an oxalate salt and an organic sodium salt and contains an oxalate \[({2^ - })\].
Complete step by step answer:
Sodium format or HCOONa, is the sodium salt of formic acid HCOOH. It usually appears as a white deliquescent powder. On heating of sodium format, decomposition reaction occurs. As a result of this reaction sodium oxalate is formed and hydrogen is released.

When sodium formate is heated at \[220 - {360^0}C\], a melt is formed, for getting Sodium oxalate, which is then spread thinly as a liquid over hot surfaces heated to a temperature between \[360 - {440^0}C\], where the decomposition to sodium oxalate takes place in 2 to 3 seconds with a quantitative yield conversion of format to oxalate.
If sodium formate is heated rapidly at 400 to \[{420^0}C\], the reaction time of thermal decomposition is much reduced and in case this optimum temperature is maintained, there is maximum production of sodium oxalate.
Therefore, the correct answer is option (B).
Note: Sodium formate is heated rapidly at 400 to \[{420^0}C\] that is overheating of sodium format in order to maximize the product, sodium oxalate (further decomposition of sodium oxalate), as possible to reduce the reaction time with optimum temperature of thermal decomposition. Overheating may cause decomposition of sodium oxalate.
Sodium oxalate is an organic sodium salt which consists of sodium ions and oxalate ions in a ratio of \[2:1\]. It plays the role of a poison as well as a reducing agent. It is an oxalate salt and an organic sodium salt and contains an oxalate \[({2^ - })\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




