
When sodium benzene sulphonate is fused with sodium hydroxide (solid), the product formed is:
(a)- Benzene
(b)- Phenol
(c)- Benzene triphenol
(d)- None of these
Answer
513.3k+ views
Hint: The aromatic compound in which the benzene carbon atom is attached to the group $S{{O}_{3}}Na$ is the sodium Benzene sulfonate. The by-products are water and sodium sulfite if sodium benzene sulfonate is treated with sodium hydroxide. When the sodium benzene sulphonate reacts with sodium hydroxide, a two-step reaction takes place.
Complete answer:
The aromatic compound in which the benzene carbon atom is attached to the group $S{{O}_{3}}Na$ is the sodium Benzene sulfonate. Below the structure of sodium benzene sulphonate is given:
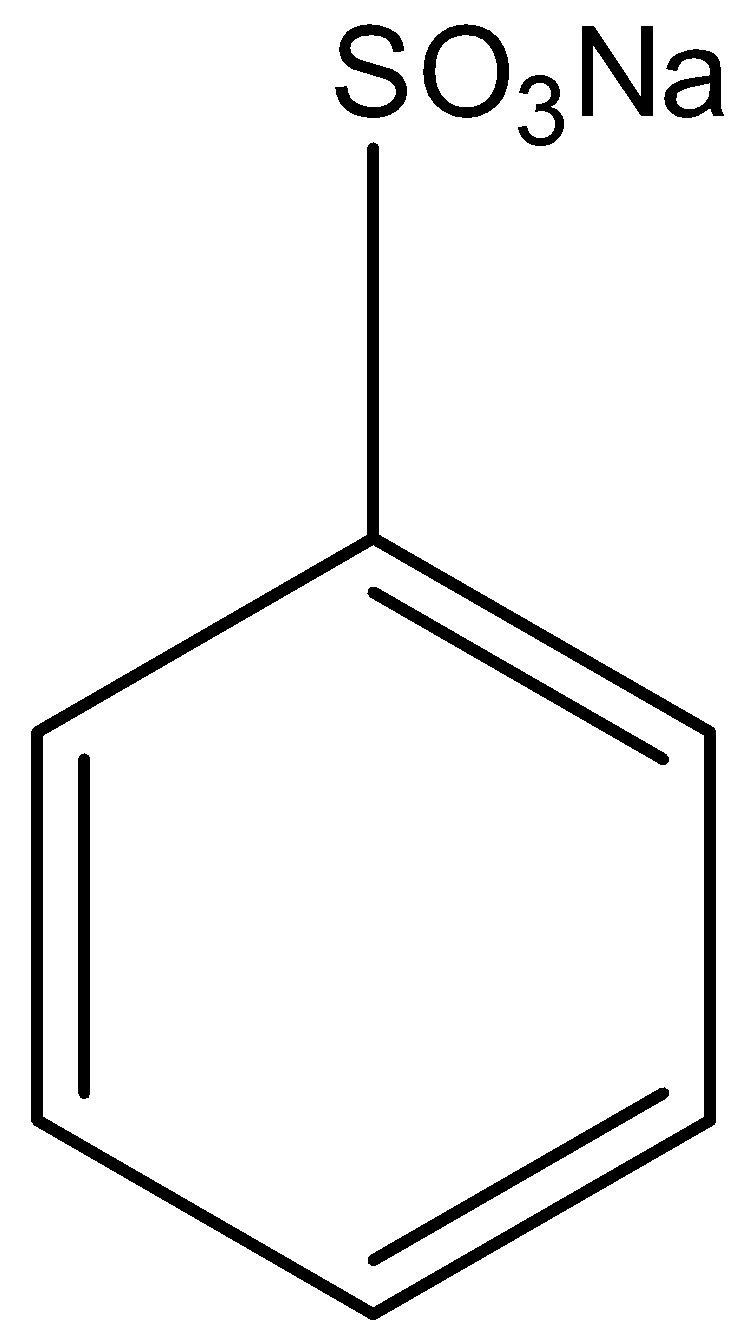
First, the sodium benzene sulphonate is treated with sodium hydroxide (solid), then the sodium sulfonate present on the benzene is replaced with the sodium oxide group. This will lead to the formation of sodium phenoxide and the by-products in the reaction are sodium sulfite and water. Next, the formed sodium phenoxide is treated with water which will replace the sodium atom with the hydrogen atom. This reaction involves acidifying Phenol forming sodium phenoxide. This reaction is only possible when acid is present. The two-step reaction is given below:
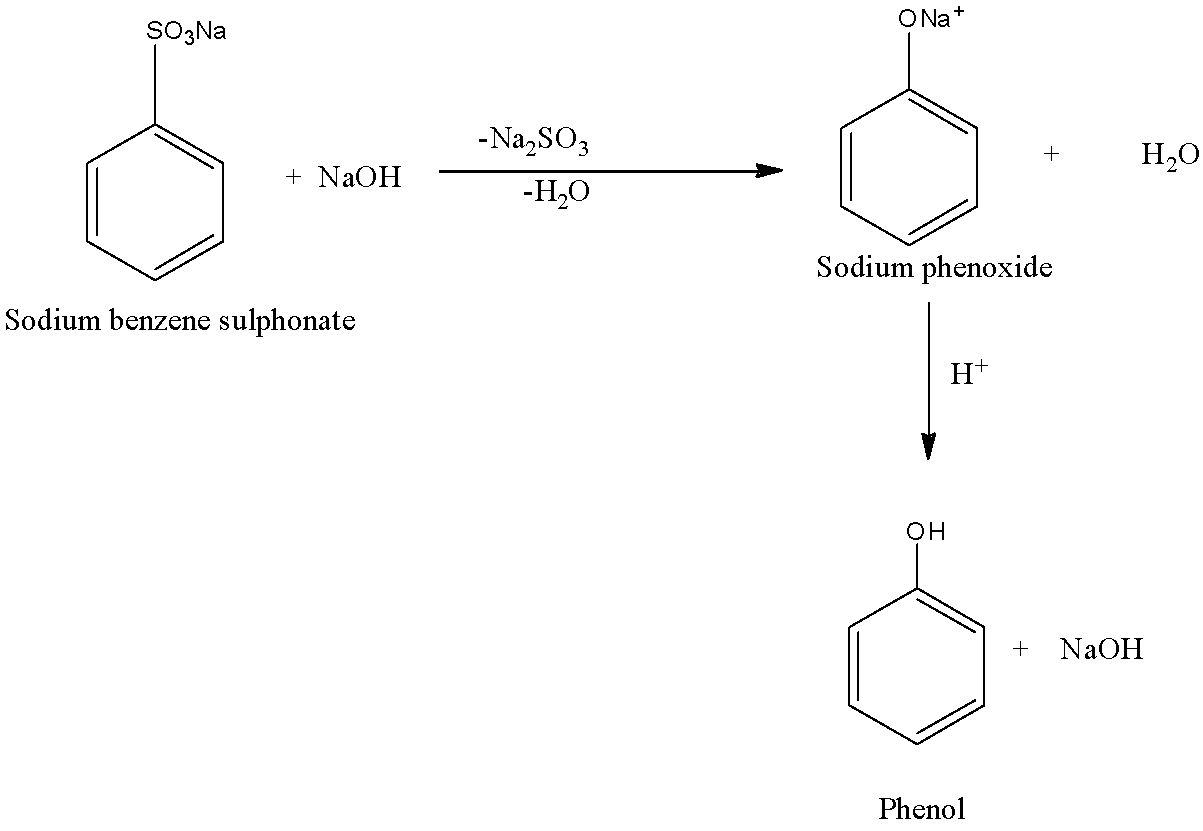
As we can see that the final product in the above reaction is the formation of phenol and sodium hydroxide is formed as the by-product.
Therefore, the correct answer is an option (b)- Phenol.
Note: It is one of the main processes in phenol production and is mainly monitored in the lab. The reaction temperature is 573-623 K. Benzene sulfonic acid sodium benzene sulphonate can be easily produced.
Complete answer:
The aromatic compound in which the benzene carbon atom is attached to the group $S{{O}_{3}}Na$ is the sodium Benzene sulfonate. Below the structure of sodium benzene sulphonate is given:

First, the sodium benzene sulphonate is treated with sodium hydroxide (solid), then the sodium sulfonate present on the benzene is replaced with the sodium oxide group. This will lead to the formation of sodium phenoxide and the by-products in the reaction are sodium sulfite and water. Next, the formed sodium phenoxide is treated with water which will replace the sodium atom with the hydrogen atom. This reaction involves acidifying Phenol forming sodium phenoxide. This reaction is only possible when acid is present. The two-step reaction is given below:

As we can see that the final product in the above reaction is the formation of phenol and sodium hydroxide is formed as the by-product.
Therefore, the correct answer is an option (b)- Phenol.
Note: It is one of the main processes in phenol production and is mainly monitored in the lab. The reaction temperature is 573-623 K. Benzene sulfonic acid sodium benzene sulphonate can be easily produced.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




