
What is the slope for an Isothermal process?
A. $\dfrac{P}{V}$
B. $-\dfrac{P}{V}$
C. Zero
D. $\infty $
Answer
589.5k+ views
Hint: Isothermal implies constant temperature. This is a thermodynamic process where the change in temperature is negligible. We will firstly write down Boyle’s Law and then will find the slope of the Isothermal process from it.
Complete step by step answer:
The thermodynamic process wherein the temperature of the system is held constant, with other parameters varying, is termed as Isothermal process. This can be denoted with a simple equation as:
$\Delta T=0$
Where, T = Temperature of the system
Although the exchange of energy, of the system with surrounding, takes place, it's rate is very slow.
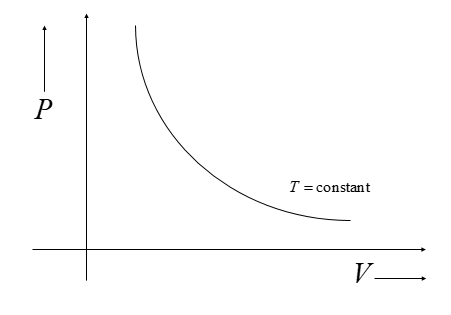
Slope of Isothermal Process:
One doesn’t need to remember the slope of the Isothermal process. It can be easily found out using
Boyle’s Law and simple mathematics.
According to Boyle’s Law:
$PV=k$ (at a given temperature) ------(i)
Now, we need to get the slope of the Isothermal process in the PV graph. To get the slope, all we need
to differentiate this equation.
We know,
$d(xy)=xdy+ydx$ -----(ii)
Here, ‘d’ is used for very small changes.
But, the change here is not very small. Hence we will use ‘$\Delta $‘ instead of ‘d’.
Differentiating equation (i)
$P\Delta V+V\Delta P$
$\Rightarrow \dfrac{\Delta P}{\Delta V}=-\dfrac{P}{V}$
So, the correct answer is “Option B”.
Note:
1.)Do not get confused between Isothermal process and adiabatic process.
2.)Adiabatic process is a thermodynamic process which prohibits any change in the energy of the system, i.e., $\Delta Q=0$. In adiabatic processes there might be change in temperature, but the net energy exchange of the system must be necessarily zero.
On the other hand, in an isothermal process the energy exchange of the system with its surrounding might not be zero.
Complete step by step answer:
The thermodynamic process wherein the temperature of the system is held constant, with other parameters varying, is termed as Isothermal process. This can be denoted with a simple equation as:
$\Delta T=0$
Where, T = Temperature of the system
Although the exchange of energy, of the system with surrounding, takes place, it's rate is very slow.

Slope of Isothermal Process:
One doesn’t need to remember the slope of the Isothermal process. It can be easily found out using
Boyle’s Law and simple mathematics.
According to Boyle’s Law:
$PV=k$ (at a given temperature) ------(i)
Now, we need to get the slope of the Isothermal process in the PV graph. To get the slope, all we need
to differentiate this equation.
We know,
$d(xy)=xdy+ydx$ -----(ii)
Here, ‘d’ is used for very small changes.
But, the change here is not very small. Hence we will use ‘$\Delta $‘ instead of ‘d’.
Differentiating equation (i)
$P\Delta V+V\Delta P$
$\Rightarrow \dfrac{\Delta P}{\Delta V}=-\dfrac{P}{V}$
So, the correct answer is “Option B”.
Note:
1.)Do not get confused between Isothermal process and adiabatic process.
2.)Adiabatic process is a thermodynamic process which prohibits any change in the energy of the system, i.e., $\Delta Q=0$. In adiabatic processes there might be change in temperature, but the net energy exchange of the system must be necessarily zero.
On the other hand, in an isothermal process the energy exchange of the system with its surrounding might not be zero.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




