
What is the slagging process? Give one example.
Answer
507.6k+ views
Hint :Ores are extracted from the earth and contain a lot of minerals depending on the type of ore. But one problem of Ore is that they are not pure. So the elements that we obtain by mining an ore have to be refined or purified in order to get very pure substances that we desire. One of the processes by which we can do this is known as slagging out.
Complete Step By Step Answer:
Impurities of any kind that are present in the ore can be removed by using slags. By using a suitable flux we can combine the impurities to produce slag that can later be removed. This process is called slagging out.
Impurities present in the ore + Flux $ \to $ Slag
Slags are left over materials that are not desirable in the final product. They usually consist of metal oxides and silicon dioxide or metal sulfides.
One of the most prominent elements that requires slagging is iron and the process of slagging can be well explained using Iron ore purification.
Let us consider that $ Si{O_2} $ is the impurity that is present in the ore. We can add a basic flux such as limestone (At high temperature it becomes lime, $ CaO $ ) to aid in the process of slagging out.
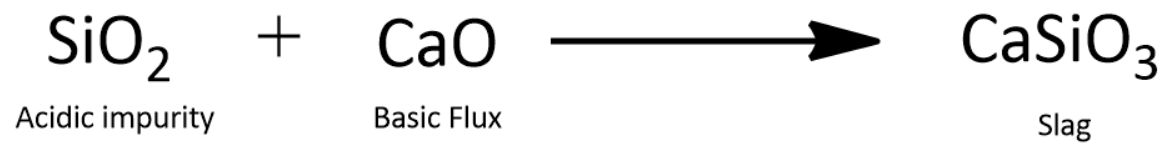
Slag being very light can be skimmed off from the surface of the molten metal.
Note :
The choice of flux depends on the type of impurity present in the ore.If the impurity present is acidic then we will use basic flux and if the impurity is basic like $ FeO $ we use acidic flux such as $ Si{O_2} $ . Flux is usually added to the ore during the reduction of the oxide in smelting
Complete Step By Step Answer:
Impurities of any kind that are present in the ore can be removed by using slags. By using a suitable flux we can combine the impurities to produce slag that can later be removed. This process is called slagging out.
Impurities present in the ore + Flux $ \to $ Slag
Slags are left over materials that are not desirable in the final product. They usually consist of metal oxides and silicon dioxide or metal sulfides.
One of the most prominent elements that requires slagging is iron and the process of slagging can be well explained using Iron ore purification.
Let us consider that $ Si{O_2} $ is the impurity that is present in the ore. We can add a basic flux such as limestone (At high temperature it becomes lime, $ CaO $ ) to aid in the process of slagging out.
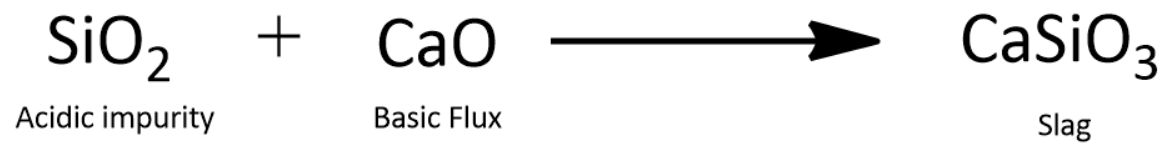
Slag being very light can be skimmed off from the surface of the molten metal.
Note :
The choice of flux depends on the type of impurity present in the ore.If the impurity present is acidic then we will use basic flux and if the impurity is basic like $ FeO $ we use acidic flux such as $ Si{O_2} $ . Flux is usually added to the ore during the reduction of the oxide in smelting
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




