
How many single bonds are there in tryptophan?
Answer
557.7k+ views
Hint: In this type of question, we should know the structure of tryptophan which is a basic amino acid. To count the number of sigma bonds, we should be clear of our idea about sigma bonds. Sigma bonds are the first bonds which are formed between two atoms.
Complete step-by-step answer:
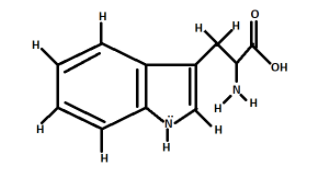
As we can see from the diagram, there are two rings in tryptophan.
We will start counting sigma bonds from the left most ring i.e., the benzene ring ${C_6}{H_6}$.
There are six C-C sigma bonds in the benzene ring and four C-H bonds in the benzene ring.
Total number of single bonds in the first ring is equal to ten$\left( {10} \right)$.
Now, coming to the second ring i.e., the pyrrole ring.
There are two C-C sigma bonds, two C-N sigma bonds, one C-H sigma bond, one N-H sigma bond.
Total number of sigma bonds in the second ring is equal to six$(6)$.
Now, coming to the aliphatic chain.
There are three C-C sigma bonds, three C-H bonds, one C-N bond, two N-H bonds, two C-O Bonds, one O-H bond.
Total number of sigma bonds in aliphatic ring is equal to twelve$\left( {12} \right)$
Total number of sigma bonds in tryptophan is equal to $10 + 6 + 12 = 28$
Hence the correct answer is 28.
Note: It has to be noted that sigma bonds are the sigma bonds in a molecule. We should always keep this in mind that double bonds and triple bonds also constitute one sigma bond each. While doing these types of questions, you should draw the structure of the compound carefully i.e., make each and every sigma bond in the structure only.
Complete step-by-step answer:

As we can see from the diagram, there are two rings in tryptophan.
We will start counting sigma bonds from the left most ring i.e., the benzene ring ${C_6}{H_6}$.
There are six C-C sigma bonds in the benzene ring and four C-H bonds in the benzene ring.
Total number of single bonds in the first ring is equal to ten$\left( {10} \right)$.
Now, coming to the second ring i.e., the pyrrole ring.
There are two C-C sigma bonds, two C-N sigma bonds, one C-H sigma bond, one N-H sigma bond.
Total number of sigma bonds in the second ring is equal to six$(6)$.
Now, coming to the aliphatic chain.
There are three C-C sigma bonds, three C-H bonds, one C-N bond, two N-H bonds, two C-O Bonds, one O-H bond.
Total number of sigma bonds in aliphatic ring is equal to twelve$\left( {12} \right)$
Total number of sigma bonds in tryptophan is equal to $10 + 6 + 12 = 28$
Hence the correct answer is 28.
Note: It has to be noted that sigma bonds are the sigma bonds in a molecule. We should always keep this in mind that double bonds and triple bonds also constitute one sigma bond each. While doing these types of questions, you should draw the structure of the compound carefully i.e., make each and every sigma bond in the structure only.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




