
Silicones are synthetic polymers containing repeated ${{R}_{2}}SiO$ units. Since the empirical formula is similar to that of a ketone $({{R}_{2}}CO)$, the name silicone has been given to these materials. Silicones can be made into oils, rubbery elastomers, and resins. They find a variety of applications due to their chemical inertness, water-repelling nature, heat-resistance, and good electrical insulating property. Commercial silicon polymers are usually methyl derivatives and to a lesser extent, phenyl derivatives and are synthesized by the hydrolysis of ${{R}_{2}}SiC{{l}_{2}}$. Consider R = methyl(Me) of phenyl(Ph).
If we mix $M{{e}_{3}}SiCl$ with $M{{e}_{2}}SiC{{l}_{2}}$, we get silicones of the type:
A.
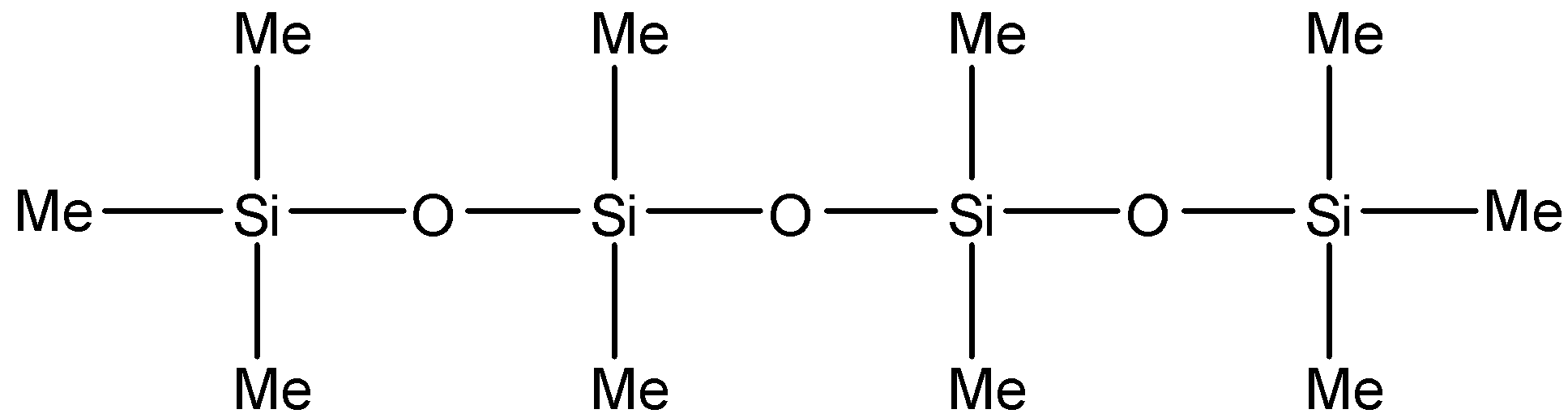
B.
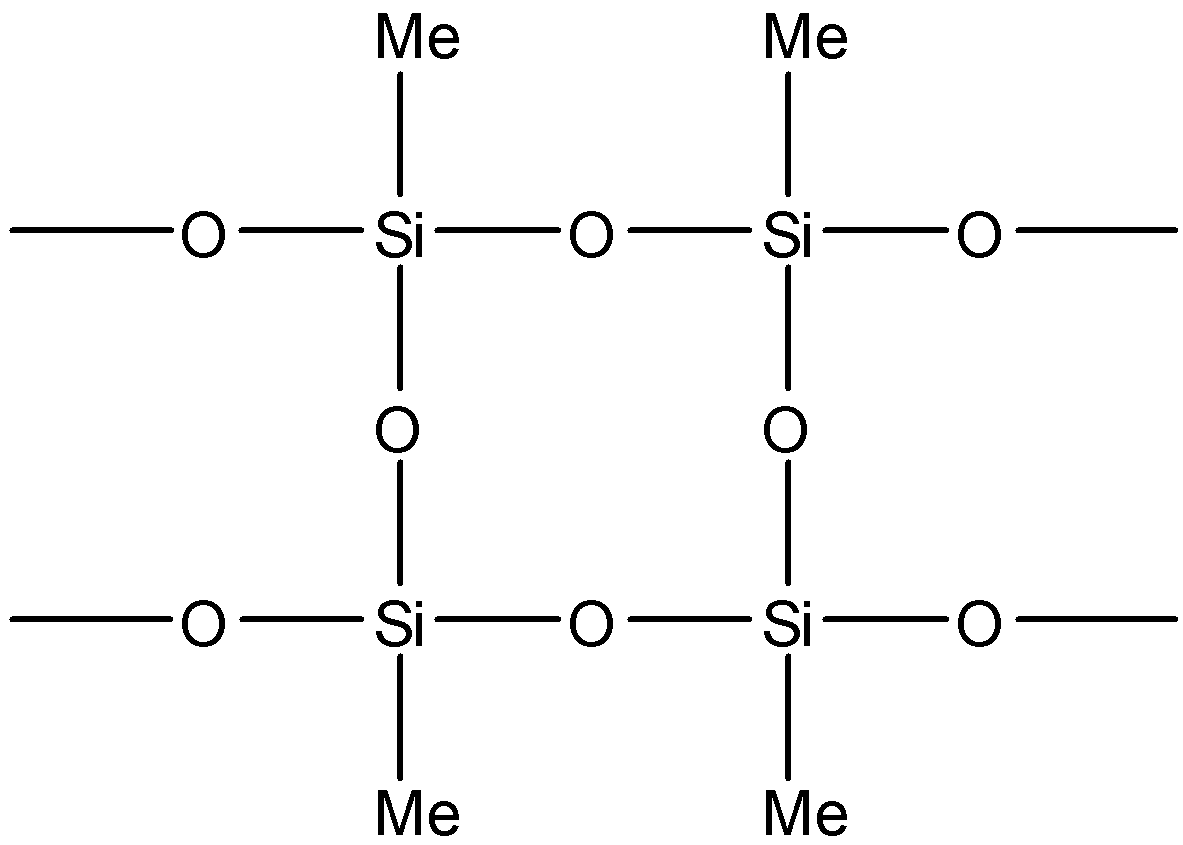
C. Both of the above
D. None of the above


Answer
590.1k+ views
Hint: Think about at what sites the hydrolysis is going to occur, consider both the types of bonds that are seen in the given alkyl silicon chlorides. Determine where the hydrolytic cleavage will occur and construct the molecule according to that.
Complete step by step solution:
In these molecules, when the water molecules react with the alkyl silicon chloride, the lone pair on the oxygen atom in water attacks the silicon atom and displaces a chlorine atom. One of the hydrogen atoms attached to this oxygen atom acts as a good leaving group and combines with the displaced hydrogen. Thus, now, a hydroxyl group is seen to be attached to silicon rather than a chlorine group. This process occurs for all the chlorine atoms that are attached to silicon but do not affect the alkyl groups at all. The reaction to this step will be as follows:
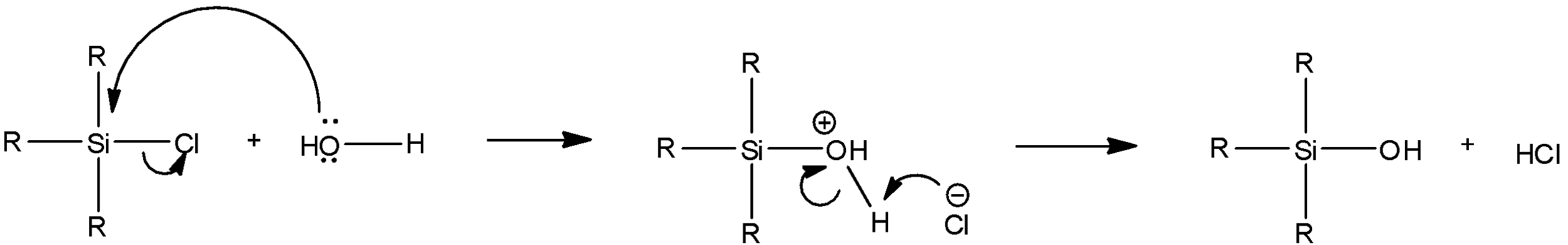
This reaction is continued for all the chlorine atoms that may be bonded to the silicon atom.
After this, a dehydration/condensation reaction takes place to form the polymer silicone. All the sites which have a hydroxyl group attached will lose either the hydroxyl group or a hydrogen proton. So, a silicone polymer will be formed. The reaction considering the two given compounds from the question is:
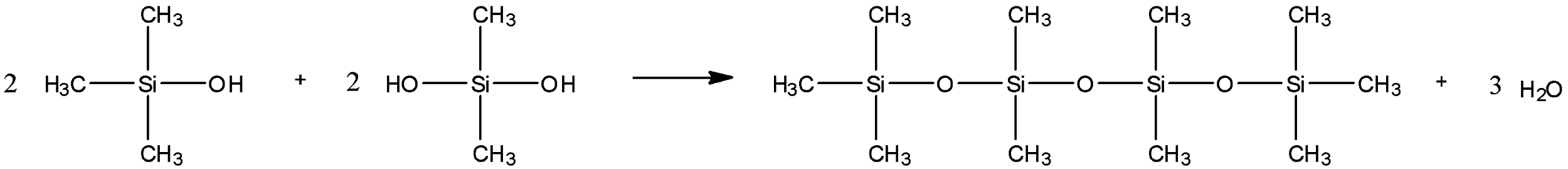
Hence, the answer to this question is option A.
Note: Note that the product mentioned in option B will not be formed since it requires silicon to be attached to at least three oxygen atoms. This means that three hydroxyl groups have to be present in the molecule before the condensation reaction. These hydroxyl groups will have replaced three chlorine atoms. But the reactants are given to us all have one or two chlorine atoms. So, it is not possible for the product in option B to be formed.
Complete step by step solution:
In these molecules, when the water molecules react with the alkyl silicon chloride, the lone pair on the oxygen atom in water attacks the silicon atom and displaces a chlorine atom. One of the hydrogen atoms attached to this oxygen atom acts as a good leaving group and combines with the displaced hydrogen. Thus, now, a hydroxyl group is seen to be attached to silicon rather than a chlorine group. This process occurs for all the chlorine atoms that are attached to silicon but do not affect the alkyl groups at all. The reaction to this step will be as follows:

This reaction is continued for all the chlorine atoms that may be bonded to the silicon atom.
After this, a dehydration/condensation reaction takes place to form the polymer silicone. All the sites which have a hydroxyl group attached will lose either the hydroxyl group or a hydrogen proton. So, a silicone polymer will be formed. The reaction considering the two given compounds from the question is:

Hence, the answer to this question is option A.
Note: Note that the product mentioned in option B will not be formed since it requires silicon to be attached to at least three oxygen atoms. This means that three hydroxyl groups have to be present in the molecule before the condensation reaction. These hydroxyl groups will have replaced three chlorine atoms. But the reactants are given to us all have one or two chlorine atoms. So, it is not possible for the product in option B to be formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




