
$SiH_{3})_{3}N$ is:
A.Planar
B.Pyramidal
C.Octahedral
D.Tetrahedral
Answer
541.5k+ views
>Hint:To obtain the solution of the question, we need to refer to the valence bond theory which explains the structure and magnetic properties of coordination compounds.
Complete step-by-step answer:- The compound given in question, has $p\pi \ -\ p\pi$ bond present. From the molecular formula we get to know that the concept of back bonding is followed here.
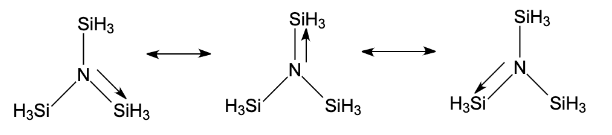
- From the structure of the given compound, we know that silicon has vacant d-orbitals. The lone pair on N provides vacant d-orbitals of Silicon with the required lone pair of electrons. Hence, $N-SiH_{3}$ bond achieves a partial double bond character that is, its hybridization changes and becomes $sp^{2}$which refers to the trigonal planar shape of the molecule.
- Now, with reference to the concept of back bonding as a sort of resonance between lone pair of electrons and the other atom with a vacant d or p-orbital present in their central atom sulfur. Hence, gives a planar shape.
Therefore, the correct option is (A).
Note:Valence bond theory tells us that the metal ion or atom under the influence of ligands uses (n-1) d, ns, np, and d orbitals for hybridization. This theory yields a set of equivalent orbitals that define geometry in terms of octahedral, tetrahedral, square planar etc. These hybridized orbitals can also overlap with the ligand orbitals to donate electron pairs for bonding.
Complete step-by-step answer:- The compound given in question, has $p\pi \ -\ p\pi$ bond present. From the molecular formula we get to know that the concept of back bonding is followed here.

- From the structure of the given compound, we know that silicon has vacant d-orbitals. The lone pair on N provides vacant d-orbitals of Silicon with the required lone pair of electrons. Hence, $N-SiH_{3}$ bond achieves a partial double bond character that is, its hybridization changes and becomes $sp^{2}$which refers to the trigonal planar shape of the molecule.
- Now, with reference to the concept of back bonding as a sort of resonance between lone pair of electrons and the other atom with a vacant d or p-orbital present in their central atom sulfur. Hence, gives a planar shape.
Therefore, the correct option is (A).
Note:Valence bond theory tells us that the metal ion or atom under the influence of ligands uses (n-1) d, ns, np, and d orbitals for hybridization. This theory yields a set of equivalent orbitals that define geometry in terms of octahedral, tetrahedral, square planar etc. These hybridized orbitals can also overlap with the ligand orbitals to donate electron pairs for bonding.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




