
How many sigma bonds are present in toluene?
Answer
520.8k+ views
Hint:Toluene occurs naturally and it is obtained from petroleum or petrochemical processes. It is a colourless liquid and is insoluble in water. It smells like paint thinners and it is a mono-substituted colourless liquid which consists of a methyl group attached to a phenyl group.
Complete answer:
Toluene is a clear and colourless liquid with a benzene like odour. The chemical formula of toluene is ${C_6}{H_5}C{H_3}$. It is used as a solvent in many products and is also used in nail polish removers and glues. It has many applications in different branches of industry. The structure of the compound toluene is as shown below:
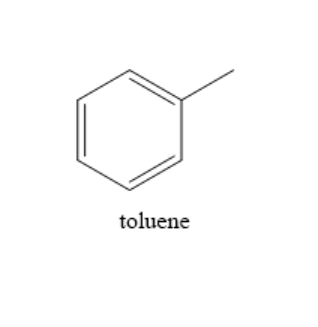
From the structure of toluene, we can observe that the benzene ring comprises twelve sigma bonds and three pi bonds and the substituent group methyl has three sigma bonds.
Therefore, toluene has $15$ sigma bonds and $3$pi bonds.
Note:
Toluene is generally found in crude oil. It is also a by-product in the production of gasoline. Also, it is obtained as a by-product in the production of coke from coal. The production of Toluene at industrial level is inexpensive. It is more reactive to electrophiles than benzene. Due to the greater part of the $C{H_3}$ group than the electron-releasing properties, it reacts with normal fragrant in the same position.
Complete answer:
Toluene is a clear and colourless liquid with a benzene like odour. The chemical formula of toluene is ${C_6}{H_5}C{H_3}$. It is used as a solvent in many products and is also used in nail polish removers and glues. It has many applications in different branches of industry. The structure of the compound toluene is as shown below:

From the structure of toluene, we can observe that the benzene ring comprises twelve sigma bonds and three pi bonds and the substituent group methyl has three sigma bonds.
Therefore, toluene has $15$ sigma bonds and $3$pi bonds.
Note:
Toluene is generally found in crude oil. It is also a by-product in the production of gasoline. Also, it is obtained as a by-product in the production of coke from coal. The production of Toluene at industrial level is inexpensive. It is more reactive to electrophiles than benzene. Due to the greater part of the $C{H_3}$ group than the electron-releasing properties, it reacts with normal fragrant in the same position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




