
How many sigma and pi bonds are there in the given molecule?
\[H - C \equiv C - C{H_2} - CH = C{H_2}\]
A. There are 3 pi bonds and 10 sigma bonds
B. There are 12 sigma bonds and 4 pi bonds
C. There are 2 pi bonds and 4 sigma bonds
D. There are 8 sigma bonds and 2 pi bonds
Answer
583.2k+ views
Hint: Sigma bonds are included in single bonds as well in the non-single bonds. Pi bonds are included in the double or triple bond.
Complete step by step answer:
We know that single bond \[\left( {{\text{C}} - {\text{H}}} \right)\] includes one sigma bond and double\[\left( {{\text{C}} = {\text{H}}} \right)\] and triple \[\left( {{\text{C}} \equiv {\text{H}}} \right)\] bonds consists of one sigma bond and the remaining bonds are pi bonds. Let us number the sigma bonds and the pi bonds in the given compound.
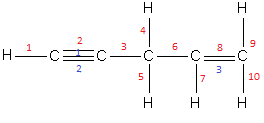
The blue numbers indicate pi bonds and the red numbers indicates sigma bonds.
Therefore, in the compound, \[H - C \equiv C - C{H_2} - CH = C{H_2}\] there exist 10 sigma bonds and 3 pi bonds.
So, we can conclude that out of the given four options, A is the correct option. B, C and D are incorrect options.
Additional information:
A sigma bond and a pi bond are formed by the overlapping of atomic orbitals. The end to end atomic orbital overlapping results in the formation of a sigma bond and the side to side overlap of the atomic orbitals generates the pi bond. We can show the orbital overlap in both the bonds in the following way.
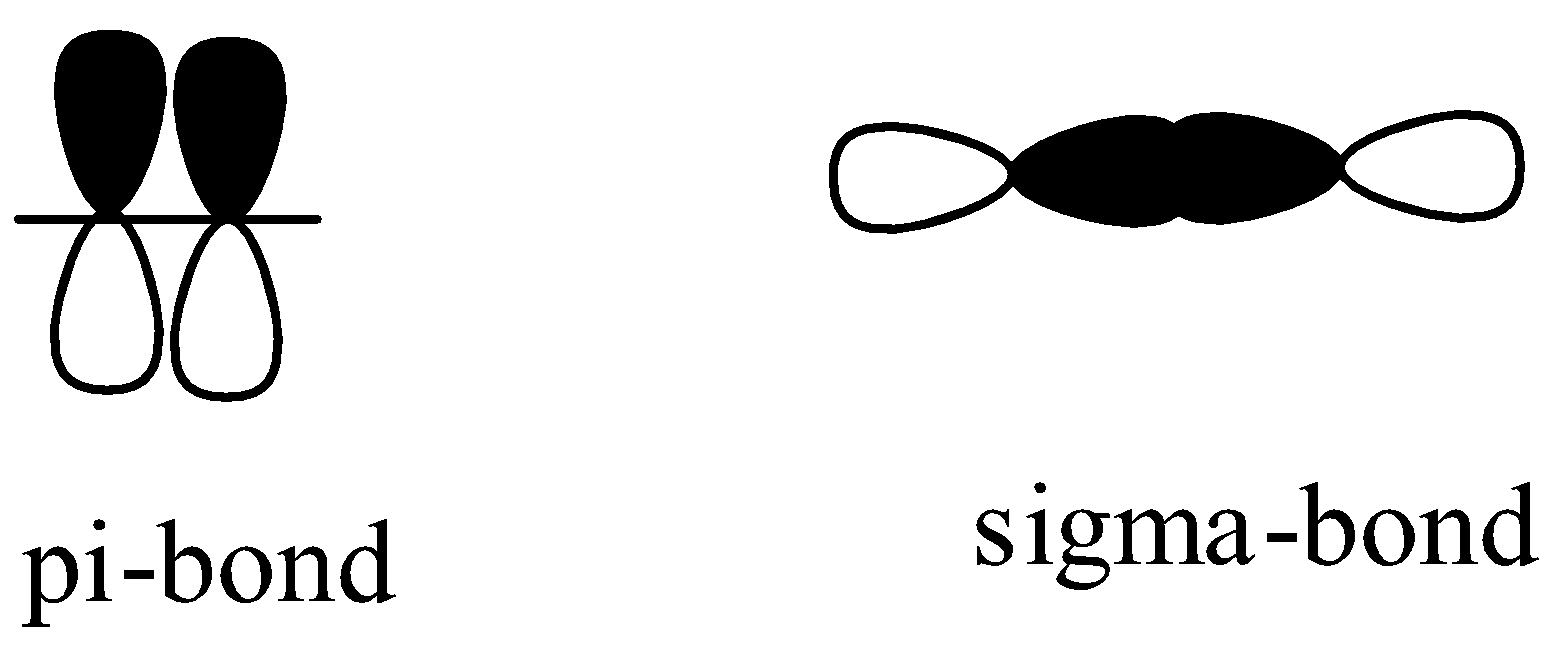
Note:Sigma bonds are stronger than pi bonds. Pi bonds are considered to be weak bonds. A bond is considered to be a strong bond if they include both the pi bond and the sigma bond together.
Complete step by step answer:
We know that single bond \[\left( {{\text{C}} - {\text{H}}} \right)\] includes one sigma bond and double\[\left( {{\text{C}} = {\text{H}}} \right)\] and triple \[\left( {{\text{C}} \equiv {\text{H}}} \right)\] bonds consists of one sigma bond and the remaining bonds are pi bonds. Let us number the sigma bonds and the pi bonds in the given compound.

The blue numbers indicate pi bonds and the red numbers indicates sigma bonds.
Therefore, in the compound, \[H - C \equiv C - C{H_2} - CH = C{H_2}\] there exist 10 sigma bonds and 3 pi bonds.
So, we can conclude that out of the given four options, A is the correct option. B, C and D are incorrect options.
Additional information:
A sigma bond and a pi bond are formed by the overlapping of atomic orbitals. The end to end atomic orbital overlapping results in the formation of a sigma bond and the side to side overlap of the atomic orbitals generates the pi bond. We can show the orbital overlap in both the bonds in the following way.

Note:Sigma bonds are stronger than pi bonds. Pi bonds are considered to be weak bonds. A bond is considered to be a strong bond if they include both the pi bond and the sigma bond together.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




