
How many sigma and pi bonds are present in benzene?
Answer
526.8k+ views
Hint: In benzene every carbon is fortified with other carbon and hydrogen. Subsequently every carbon there will be two sigma bonds, one with other carbon and another is with hydrogen.
Complete step by step solution:
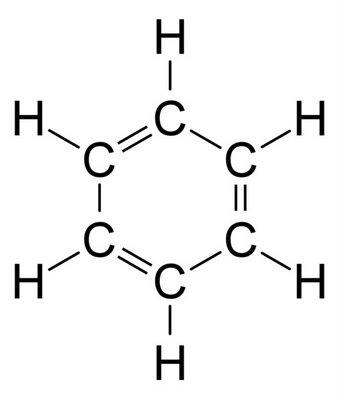
In the structure when we draw a straight line between two molecules, you would cross where the sigma bond is put. So the unavoidable issue is the place where might the second or third bond in a two fold bond or triple bond be put since you can't put two bonds in a similar spot.
These bonds are framed by the cover of the p-orbitals. In the event that you review from science, the p orbital has a nut shape. This is the thing that we call a pi bond.
Without going into a lot more detail, to address your inquiry, there is a sigma connection between each molecule in benzene. There is likewise an expansion bond called a pi bond present any place there is a twofold bond. This prompts 12 sigma bonds (remember the hydrogens) and 3 pi bonds. It ought to be noticed that the pi bonds are in a formed framework and turn around the ring which offers dependability to benzene.
Note:
At the point when we go to no. of pi bonds in every carbon in benzene there will be one hybridized orbital that will cover with other carbon pivotally to shape pi bonds. Each two carbons will cover pivotally to shape a pi bond.
Complete step by step solution:

In the structure when we draw a straight line between two molecules, you would cross where the sigma bond is put. So the unavoidable issue is the place where might the second or third bond in a two fold bond or triple bond be put since you can't put two bonds in a similar spot.
These bonds are framed by the cover of the p-orbitals. In the event that you review from science, the p orbital has a nut shape. This is the thing that we call a pi bond.
Without going into a lot more detail, to address your inquiry, there is a sigma connection between each molecule in benzene. There is likewise an expansion bond called a pi bond present any place there is a twofold bond. This prompts 12 sigma bonds (remember the hydrogens) and 3 pi bonds. It ought to be noticed that the pi bonds are in a formed framework and turn around the ring which offers dependability to benzene.
Note:
At the point when we go to no. of pi bonds in every carbon in benzene there will be one hybridized orbital that will cover with other carbon pivotally to shape pi bonds. Each two carbons will cover pivotally to shape a pi bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




