
How many sigma and pi bonds are in xylene?
Answer
489.9k+ views
Hint: Let us first understand what sigma and pi bonds are. The overlapping of atomic orbitals distinguishes sigma and pi bonds from each other. The overlapping of atomic orbitals forms covalent bonds. The head-to-head overlapping of atomic orbitals form sigma bonds, whereas the lateral overlap of two atomic orbitals forms pi bonds.This overlap happens in two ways sigma and pi bonds , resulting in two types of covalent bonds:
Complete answer:
Xylene comes in three isomers, however they all have the same number of sigma and pi bonds.
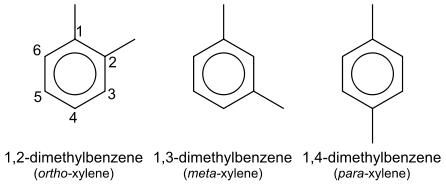
Treating the skeletal structure as all sigma bonds and adding pi bonds for each double bond in the primary resonance structure is an easy approach to count these.
Taking m-xylene,
Hexagons have six sides, resulting in six sigma bonds.
On the benzene ring, each carbon-hydrogen bond adds one bond, for a total of four.
Each methyl group's carbon-carbon bond is counted as one bond, resulting in a total of two sigma bonds. Each methyl group has three carbon-hydrogen bonds, which add up to a total of six additional bonds. As a result, we have a total of $18$ sigma bonds.
We've delocalized pi electrons over six atoms inside the benzene ring. Three pi bonds make up the main resonance structure, one for each double bond.
So, we have $18$ sigma and $3$ pi bonds.
Note:
It can be noted that xylene is a type of organic chemical. It's also known as Xylol or dimethylbenzene. It is one of the three dimethylbenzene isomers. It is composed of a central benzene ring with two methyl groups attached as substituent. It's a clear, colourless liquid that's combustible. Catalytic reforming and coal carbonization are both used to make it. It's also found in crude oil, aircraft fuel, and gasoline.
Complete answer:
Xylene comes in three isomers, however they all have the same number of sigma and pi bonds.

Treating the skeletal structure as all sigma bonds and adding pi bonds for each double bond in the primary resonance structure is an easy approach to count these.
Taking m-xylene,
Hexagons have six sides, resulting in six sigma bonds.
On the benzene ring, each carbon-hydrogen bond adds one bond, for a total of four.
Each methyl group's carbon-carbon bond is counted as one bond, resulting in a total of two sigma bonds. Each methyl group has three carbon-hydrogen bonds, which add up to a total of six additional bonds. As a result, we have a total of $18$ sigma bonds.
We've delocalized pi electrons over six atoms inside the benzene ring. Three pi bonds make up the main resonance structure, one for each double bond.
So, we have $18$ sigma and $3$ pi bonds.
Note:
It can be noted that xylene is a type of organic chemical. It's also known as Xylol or dimethylbenzene. It is one of the three dimethylbenzene isomers. It is composed of a central benzene ring with two methyl groups attached as substituent. It's a clear, colourless liquid that's combustible. Catalytic reforming and coal carbonization are both used to make it. It's also found in crude oil, aircraft fuel, and gasoline.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




