
How many sigma and pi bonds are in the Lewis structure for $ CHCC{H_2}COOH $ ?
Answer
528.6k+ views
Hint: In order to answer the question, first we will write the IUPAC name of the given compound and then draw the structural formula of the given compound. And then discuss the presence of sigma and pi bonds.
Complete answer:
The structural formula and IUPAC name of the given compound is
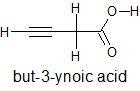
To know the no. of $ \sigma \,and\,\pi $ bonds the Lewis structure of the compound is to be drawn showing all single, double and triple bonds. In this structure
Each single bond is $ 1\sigma $ bond
Each double bond is $ 1\sigma \,and\,1\pi $ bond
Each triple bond is $ 1\sigma \,and\,2\pi $ bond
Using this information we can get the no. of $ \sigma \,and\,\pi $ bonds in the given structure
$ 7single{\text{ }}bonds \to 7\sigma bonds $
$ 1double{\text{ }}bond \to 1\sigma \,and\,1\pi \,bonds $
$ 1triple{\text{ }}bond \to 1\sigma \,and\,2\pi \,bonds $
Note:
$ 3 - butenoic acid $ is a monocarboxylic acid consisting of acetylene carrying a carboxymethyl group. It is a monocarboxylic acid and a terminal acetylenic compound. It is a conjugate acid of a $ 3 - butenoate $ .
Complete answer:
The structural formula and IUPAC name of the given compound is

To know the no. of $ \sigma \,and\,\pi $ bonds the Lewis structure of the compound is to be drawn showing all single, double and triple bonds. In this structure
Each single bond is $ 1\sigma $ bond
Each double bond is $ 1\sigma \,and\,1\pi $ bond
Each triple bond is $ 1\sigma \,and\,2\pi $ bond
Using this information we can get the no. of $ \sigma \,and\,\pi $ bonds in the given structure
$ 7single{\text{ }}bonds \to 7\sigma bonds $
$ 1double{\text{ }}bond \to 1\sigma \,and\,1\pi \,bonds $
$ 1triple{\text{ }}bond \to 1\sigma \,and\,2\pi \,bonds $
Note:
$ 3 - butenoic acid $ is a monocarboxylic acid consisting of acetylene carrying a carboxymethyl group. It is a monocarboxylic acid and a terminal acetylenic compound. It is a conjugate acid of a $ 3 - butenoate $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




