
How many sigma and pi bonds are in the HCCH molecule?
Answer
535.8k+ views
Hint: The sigma bond is shown as $\sigma -bond$ and pi bond are shown as $\pi -bond$, if all the bonds in the compound are single then there are only sigma bonds, and pi bonds are present in the double and the triple bonds. HCCH molecules are known as ethyne or acetylene.
Complete answer: If the compounds are covalent, it means that there are covalent bonds in them, and there are two types of a covalent bonds, i.e., sigma bond shown as $\sigma -bond$ and pi bond shown as $\pi -bond$.
By counting the number of bonds, we can calculate the number of sigma and pi bonds. Sigma and pi bonds are based on the type of bond present in the molecule, i.e., a single bond, double bond, and triple bond. Therefore, in single bonds, only a sigma bond is present, in the double bond one is sigma and one is pi, and in the triple bond one is a sigma bond and two are pi bonds.
HCCH molecule is known as ethyne or acetylene and it is the first member of the alkyne group, and alkynes are the compounds in which there is a triple bond. The formula of ethyne is given below:
$H-C\equiv C-H$
Since there is one triple bond in this compound, therefore, there are 2 pi bonds present. Rest there are 3 bonds present, so there are 3 sigma bonds.
The diagram is given below:
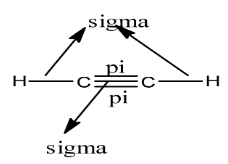
Therefore, there are 3 sigma bonds and 2 pi bonds present in HCCH.
Note: The sigma bond is a stronger bond than the pi bond because overlapping in the sigma bond is along the internuclear axis but in the pi bond there is sideways overlapping of orbitals.
Complete answer: If the compounds are covalent, it means that there are covalent bonds in them, and there are two types of a covalent bonds, i.e., sigma bond shown as $\sigma -bond$ and pi bond shown as $\pi -bond$.
By counting the number of bonds, we can calculate the number of sigma and pi bonds. Sigma and pi bonds are based on the type of bond present in the molecule, i.e., a single bond, double bond, and triple bond. Therefore, in single bonds, only a sigma bond is present, in the double bond one is sigma and one is pi, and in the triple bond one is a sigma bond and two are pi bonds.
HCCH molecule is known as ethyne or acetylene and it is the first member of the alkyne group, and alkynes are the compounds in which there is a triple bond. The formula of ethyne is given below:
$H-C\equiv C-H$
Since there is one triple bond in this compound, therefore, there are 2 pi bonds present. Rest there are 3 bonds present, so there are 3 sigma bonds.
The diagram is given below:

Therefore, there are 3 sigma bonds and 2 pi bonds present in HCCH.
Note: The sigma bond is a stronger bond than the pi bond because overlapping in the sigma bond is along the internuclear axis but in the pi bond there is sideways overlapping of orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




