
How many sigma and pi bonds are in \[Se{O_3}^{2 - }\]?
Answer
555.3k+ views
Hint: We need to understand what are sigma and pi bonds and how do we calculate them. In addition, to study about $\left( \sigma \right)$ and $\left( \Pi \right)$ bonds we need to understand molecular orbitals and molecular orbital theory. Understanding of atomic orbitals is also necessary. The first covalent bond between two atoms is always a sigma bond $\left( \sigma \right)$ . Any second or third bond is known as a pi bond. Hence, we would first need to understand the bonding in the given molecule and accordingly calculate the number of sigma and pi bonds in \[Se{O_3}^{2 - }\].
Complete step by step answer:
The first covalent bond between two atoms is always a sigma bond $\left( \sigma \right)$. Any second or third bond is known as a pi bond. Let us explain this with the help of a simple $HCN$ molecule. This molecule has one single and one triple bond given as follows $H - C \equiv N$ . As we know that the first covalent bond is always a sigma bond, the single bond between $H$ and $C$ is definitely a sigma bond and the first bond between $C$ and $N$ is a sigma bond and the remaining two are pi bonds.
The given molecule is a selenite ion \[Se{O_3}^{2 - }\] . Its skeletal structure is given below:
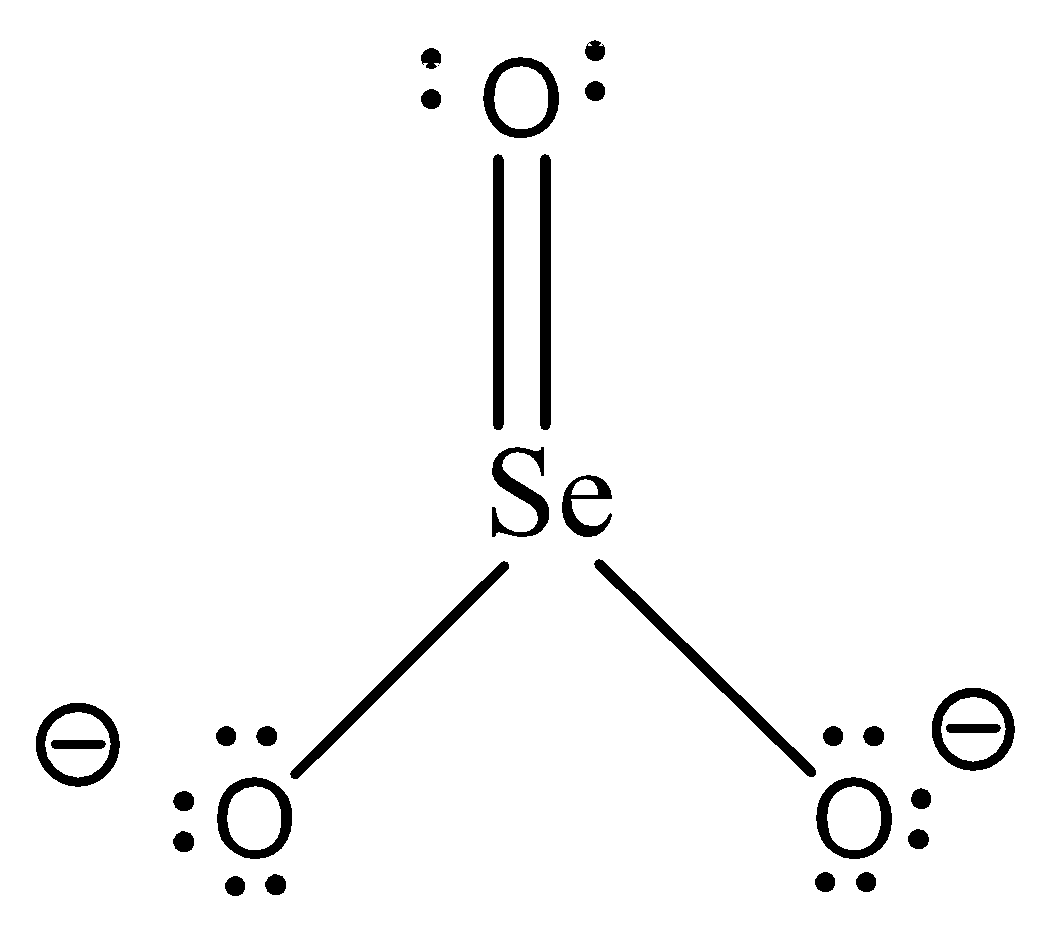
As seen in the skeletal structure, there are 2 single bonds and one double bond. Hence the 2 single bonds are sigma bonds along with the first bond of the double bond and the second bond of the double bond is a pi bond.
Hence, there are three sigma bonds and one pi bond in \[Se{O_3}^{2 - }\] .
Note: It must be noted that there is a simple trick to calculate the number of sigma bonds in a molecule. The formula for this trick is \[S = x - 1\] where $S - $ the number of sigma bonds and $x$ is the number of atoms in the molecule. The number of molecules \[Se{O_3}^{2 - }\] is $4$. According to the formula, $x = 4$ .Therefore, $S = 4 - 1 = 3$ sigma bonds and one pi bond.
Complete step by step answer:
The first covalent bond between two atoms is always a sigma bond $\left( \sigma \right)$. Any second or third bond is known as a pi bond. Let us explain this with the help of a simple $HCN$ molecule. This molecule has one single and one triple bond given as follows $H - C \equiv N$ . As we know that the first covalent bond is always a sigma bond, the single bond between $H$ and $C$ is definitely a sigma bond and the first bond between $C$ and $N$ is a sigma bond and the remaining two are pi bonds.
The given molecule is a selenite ion \[Se{O_3}^{2 - }\] . Its skeletal structure is given below:

As seen in the skeletal structure, there are 2 single bonds and one double bond. Hence the 2 single bonds are sigma bonds along with the first bond of the double bond and the second bond of the double bond is a pi bond.
Hence, there are three sigma bonds and one pi bond in \[Se{O_3}^{2 - }\] .
Note: It must be noted that there is a simple trick to calculate the number of sigma bonds in a molecule. The formula for this trick is \[S = x - 1\] where $S - $ the number of sigma bonds and $x$ is the number of atoms in the molecule. The number of molecules \[Se{O_3}^{2 - }\] is $4$. According to the formula, $x = 4$ .Therefore, $S = 4 - 1 = 3$ sigma bonds and one pi bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




